Solid-oxide fuel battery anode catalysis material containing rare earth element
A solid oxide, fuel cell technology, used in metal/metal oxide/metal hydroxide catalysts, battery electrodes, physical/chemical process catalysts, etc., can solve the loss of battery activity, electrode activity reduction, electrode structure damage, etc. problems, to achieve the effect of improving interface contact, improving output performance, and reducing polarization impedance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
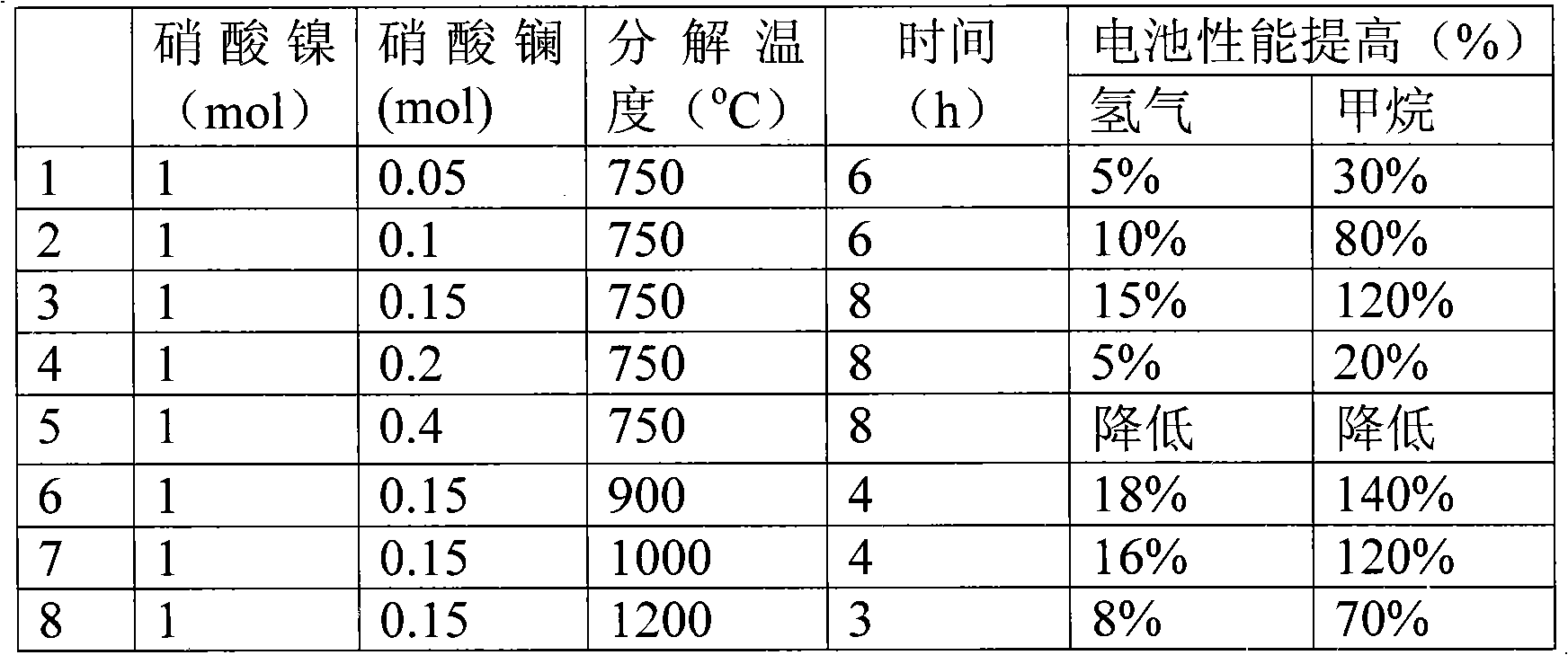
[0019] Effect of Lanthanum-modified Nickel Oxide Catalytic Materials on the Performance of Solid Oxide Fuel Cells by Nitrate Decomposition
[0020] A nitrate decomposition method is used to co-decompose the mixture of lanthanum nitrate and nickel nitrate between 700°C and 1200°C to obtain a lanthanum-modified nickel oxide electrode catalyst material.
[0021] Use lanthanum-modified nickel oxide as the anode catalyst material to mix with yttria-stabilized zirconia (8YSZ, the molar content of yttria in YSZ is 8%) (50:50 by weight) to prepare the anode, and the yttria-stabilized zirconia (8YSZ) as the electrolyte, and LSM-YSZ (50:50 by weight) as the cathode to prepare the battery.
[0022] Test conditions: at 800°C, hydrogen (80ml / min) or methane (20ml / min) is used as the anode fuel gas, and oxygen (40ml / min) is used as the cathode gas.
[0023] Table 1
[0024]
[0025] It can be seen from Table 1 that with the increase of lanthanum content, the performance of the battery ...
Embodiment 2
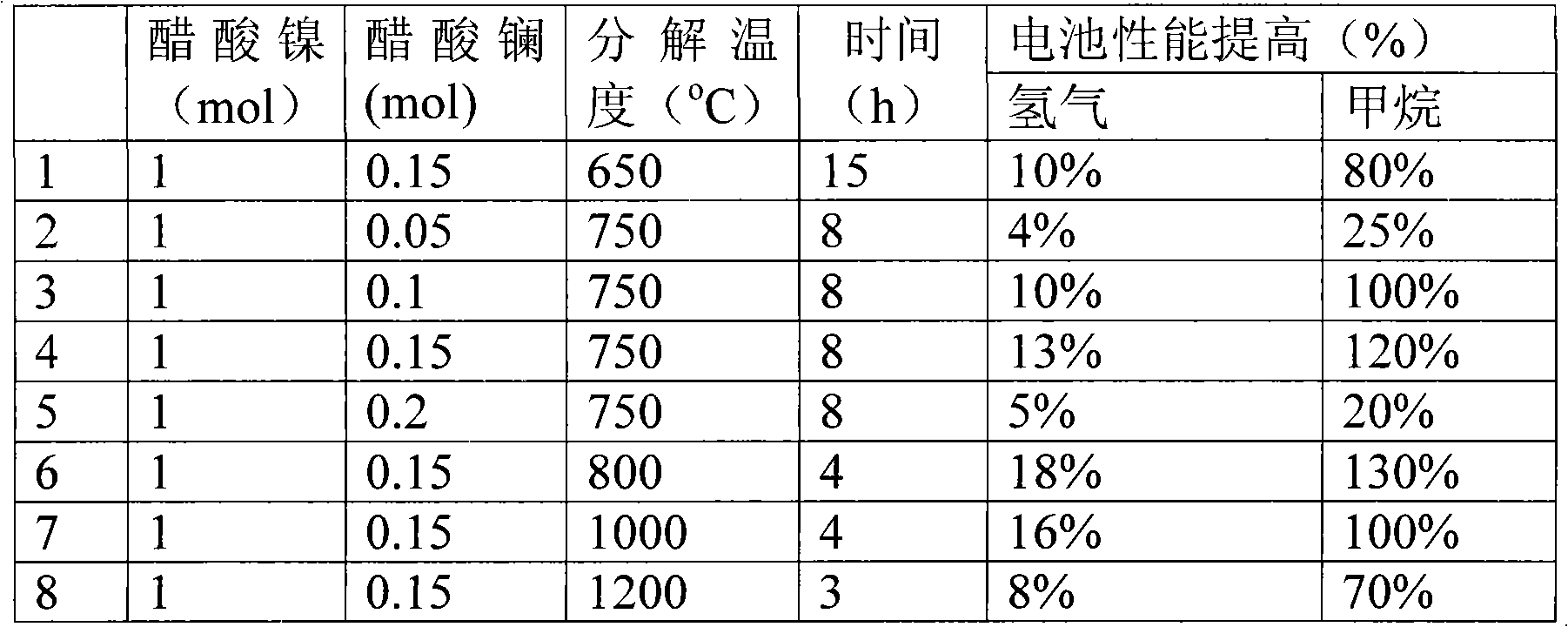
[0027] Effect of Lanthanum-modified Nickel Oxide on Battery Performance by Acetate Decomposition
[0028] The acetate decomposition method is used to co-decompose the mixture of lanthanum acetate and nickel acetate between 650°C and 1200°C to obtain a lanthanum-modified nickel oxide electrode catalytic material. The battery preparation method and test conditions are the same as in Example 1.
[0029] Table 2
[0030]
[0031] The results in Table 2 are similar to those in Table 1, indicating that a good catalyst material for solid oxide fuel cell anodes can also be obtained by using the acetate decomposition method.
Embodiment 3
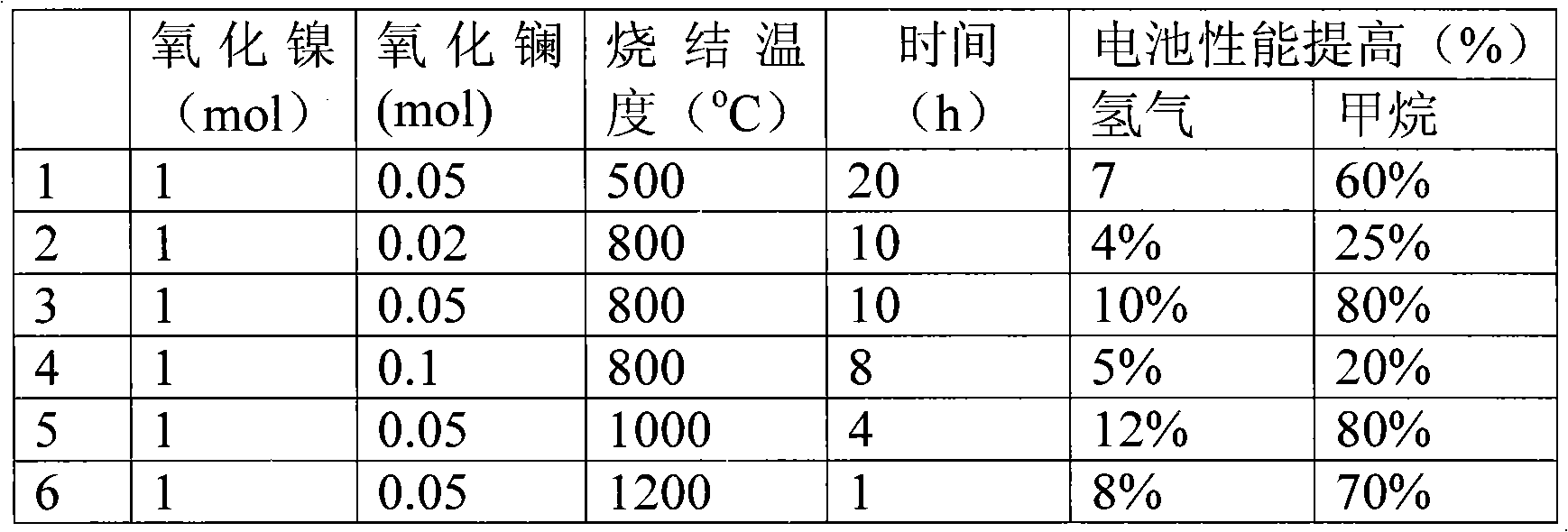
[0033] Effect of Lanthanum Oxide Modified Nickel Oxide Used in Solid Oxide Fuel Cells on Cell Performance
[0034] The lanthanum oxide and nickel oxide in different proportions are mixed and calcined at 500° C. to 1200° C. to obtain the electrode catalytic material. Using this electrode catalyst material to prepare a battery, the battery preparation method and test conditions are the same as in Example 1.
[0035] table 3
[0036]
[0037] It can be seen from Table 3 that when lanthanum oxide is used to modify nickel oxide for solid oxide fuel cells, the battery performance will also be greatly improved.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com