Novel synthesizing method for dorzolamide HCL midbody
A compound, reaction time technology, applied in the direction of organic chemistry, etc., can solve the problems of increasing process complexity, loss of yield and purity, and many reaction steps.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1 Preparation of 3-(2-mercaptothiophene) butanoic acid
[0052] Install the glass instrument, protect it with nitrogen, add 750 ml of THF, heat, add 48 g of magnesium chips, 5 g of iodine under stirring, heat to about 75 ° C and start to reflux, and slowly add 81.5 g of 2-bromothiophene dropwise under reflux and 500 milliliters of tetrahydrofuran, after the dropwise addition was completed, the solution was kept under reflux and stirred for 4 hours.
[0053] Cool the reaction solution to room temperature and stop stirring. After standing still, pour the supernatant into another three-necked flask that is also dry, under nitrogen protection, under ice bath conditions, add 38 grams of settled sulfur in three batches, and keep the temperature at - 5°C to 5°C. After the addition, the reaction system was naturally raised to room temperature (15°C to 25°C), and then kept at this temperature and stirred for 20 hours.
[0054] A mixed solution of 172 grams of crotonic a...
Embodiment 2
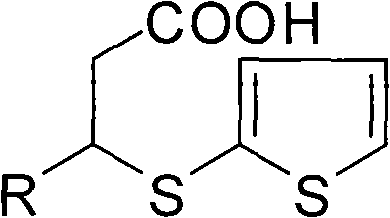
[0055] The preparation of embodiment 2 (S)-3-(2-mercaptothiophene) butanoic acid
[0056] Mix 202 grams of 3-(2-mercaptothiophene) butanoic acid and 122 grams of (-)-α-phenylethylamine in 500 milliliters of ethyl acetate, heat and stir until completely dissolved, heat and reflux for 15 hours, and cool to 10°C , crystals are precipitated, gradually cooled to -15°C under stirring, until the crystals are completely precipitated, filtered, the filter cake is repeated 3 times after the above operation, and dried to obtain a solid dissolved in water, add 300 ml of 10% NaOH solution, and heat Stir, and finally add HCl solution dropwise to adjust the pH value to 2, separate the liquid, wash with water several times, dry over anhydrous sodium sulfate, filter, and spin dry the solvent to obtain 82 g of light yellow oil with an optical purity of 98%.
Embodiment 3
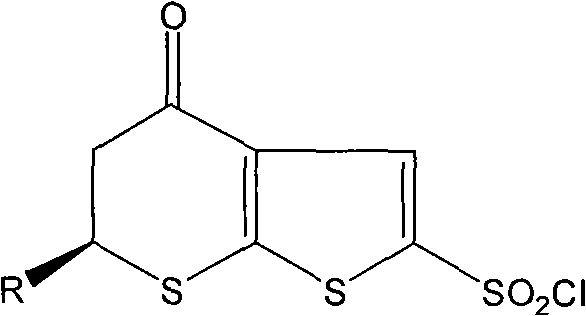
[0057] Example 3 (S)-6-methyl-2-sulfonyl chloride-5,6-dihydro-4H-thiophene[2,3b]thiopyran-4-carbonyl
[0058] Add 228 ml of fuming sulfuric acid into a three-necked flask in an ice bath to -7°C, add dropwise 82 g of (S)-3-(2-mercaptothiophene) butanoic acid under stirring, and react at room temperature for 7 hours. 200 ml of sulfoxide, heated to 37-40°C, reacted for 8 hours, cooled to -10°C, added dropwise 26 ml of tin tetrachloride in dichloromethane solution, kept the temperature not higher than 0°C during the dropwise addition, completed Then stir at 0°C for 1 hour. The reaction solution was poured into a mixture of ice and water, and 93 g of solid was obtained by filtration, with a yield of 81.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com