Red phosphor excitated by blue light and preparation thereof
A technology of red light fluorescence and blue light excitation, which is applied in the field of red light phosphor materials, can solve the problems of low efficiency and achieve the effects of simple and easy-to-operate production process, high light efficiency, and cheap and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
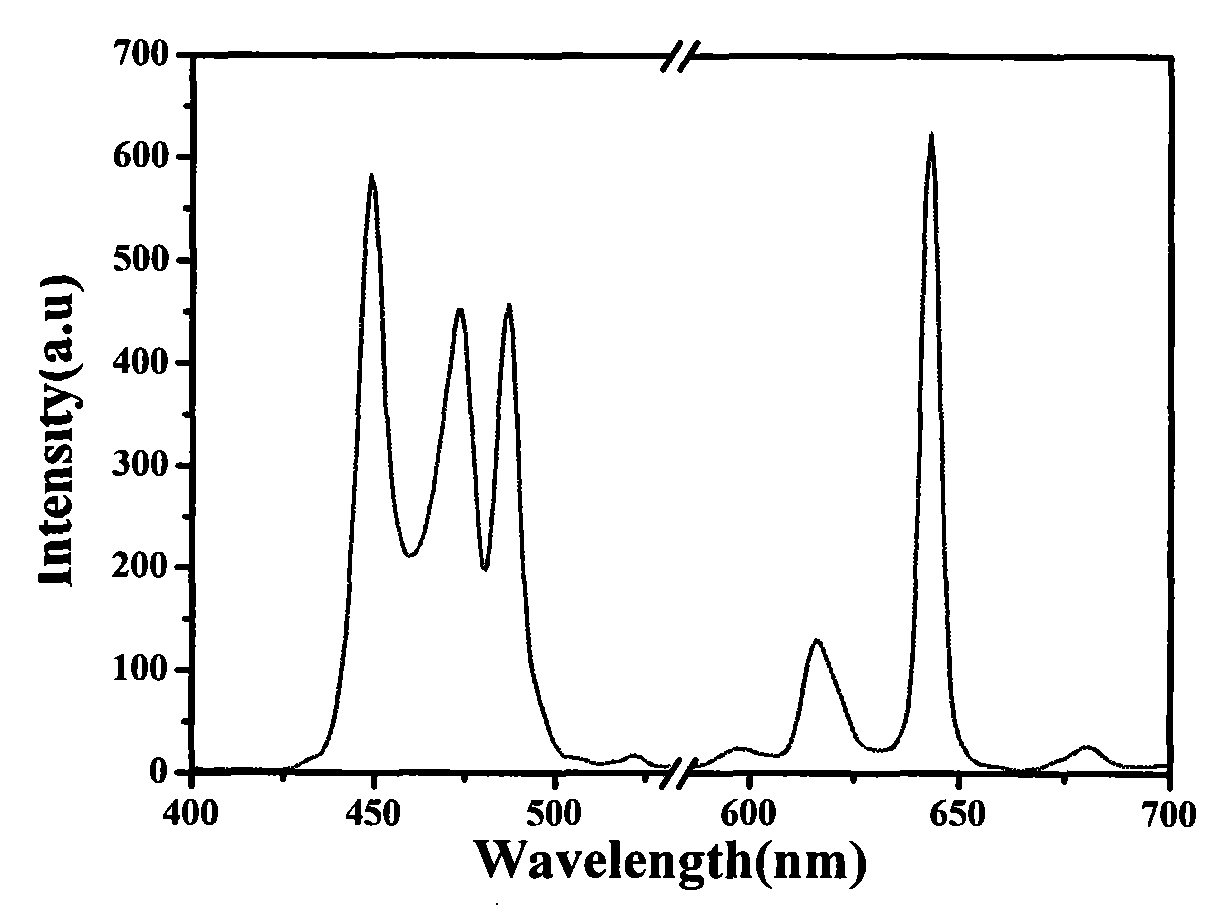
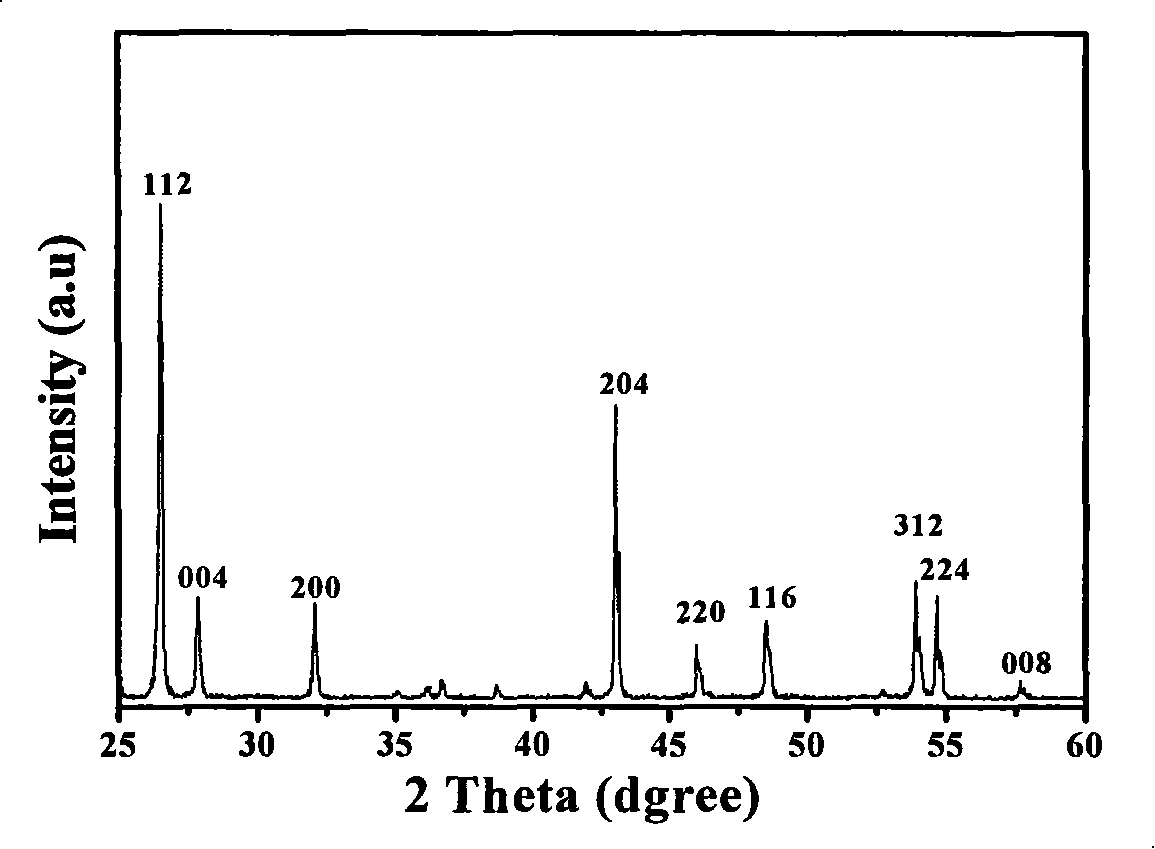
[0021] Weigh 0.01mol BaCO 3 , 0.0014mol (NH 4 ) 6 Mo 7 o 24 4H 2 O, 0.000833 mol Pr 6 o 11 Grind and mix with 0.0002mol KCl in a fume hood to obtain the precursor, put the above precursor in a muffle furnace for sintering at 900°C for 2 hours, and then grind slightly to obtain the target product. The obtained product was determined by energy dispersive X-ray spectroscopy, and its composition was BaMoO 4 :(Pr, KCl) n , n=0.02, the average particle size is 1-5um. Its fluorescence spectrum and XRD pattern are as follows figure 1 and figure 2 shown.
[0022] Fluorescence spectrum detection conditions: test with Eclipse fluorescence spectrometer, its excitation wavelength: 449nm; emission wavelength: 643nm; voltage: 440V. It can be seen from the fluorescence spectrum that the phosphor has a strong absorption of visible light in the range of 430-500nm, and a strong emission at 630-650nm.
[0023] XRD pattern detection condition: use Rigaku D / max-rC type X-ray diffracto...
Embodiment 2
[0025] Weigh 0.01mol SrCO 3 , 0.0014mol (NH 4 ) 6 Mo 7 o 24 4H 2 O, 0.0002molPr(NO 3 ) 3 ·6H 2 O and 0.0002mol KCl were ground and mixed in a fume hood to obtain a precursor, and the above precursor was sintered in a muffle furnace at 1000°C for 3 hours, and then ground slightly to obtain the target product. The resulting product was determined by energy dispersive X-ray spectroscopy, and its composition was SrMoO 4 :(Pr, KCl) n , n=0.02, the average particle size is 1-5um.
Embodiment 3
[0027] Weigh 0.01mol CaO, 0.01mol Mo(NO 3 ) 3 ·5H 2 O, 0.0000333 mol Pr 6 o 11 Grinding and mixing with 0.002mol KCl in a fume hood to obtain a precursor, putting the above precursor in a muffle furnace for sintering at 800°C for 5 hours, and then grinding a little to obtain the target product. The resulting product was determined by energy dispersive X-ray spectroscopy, and its composition was CaMoO 4 :(Pr, KCl) n , n=0.02, the average particle size is 1-5um.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com