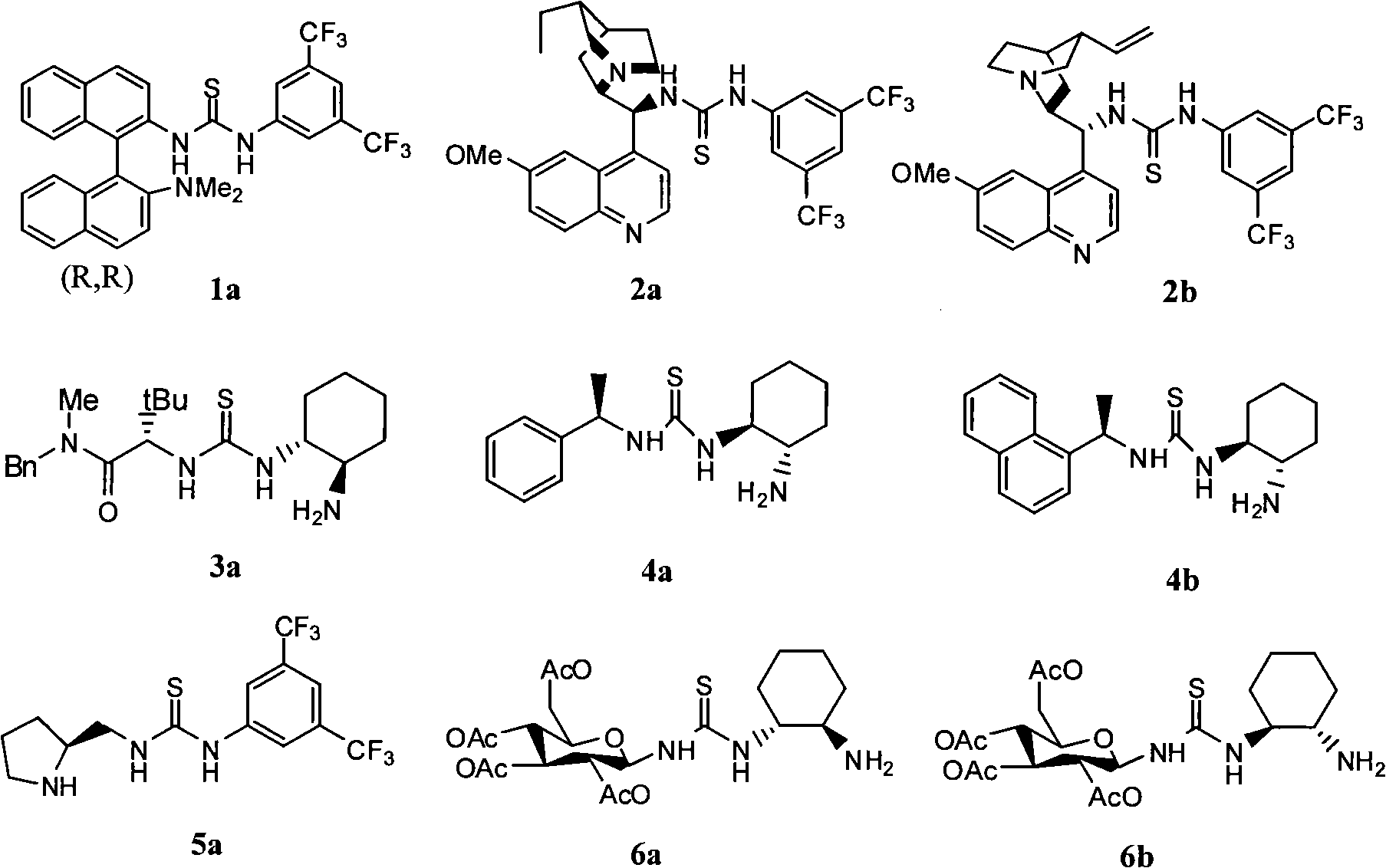
Application of dehydroabietylamine thiourea catalyst in synthesizing chiral compound with high-antipodism both-hand control
A dehydroabietic amine and thiourea catalyst technology, which is applied in the preparation of organic compounds, asymmetric synthesis, organic compound/hydride/coordination complex catalysts, etc., can solve the unreported γ-nitro-aromatic heteroketone The synthesis method of chiral compounds, poor adaptability of cyclohexanediamine, low enantioselectivity, etc., achieve the effects of easy modification and preparation, low price, easy separation and recovery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
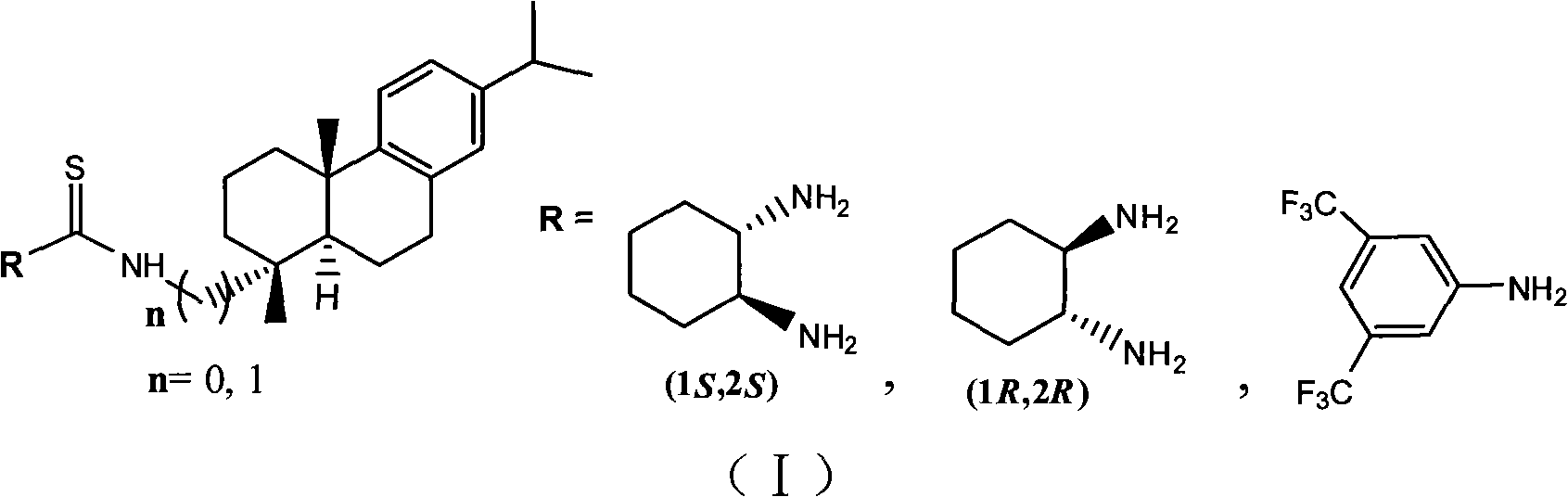
[0046] Embodiment 1: dehydroabietamine-thiourea catalyst (abbreviation: (1R, 2R)-CHDA-DHATU), [English full name 1-((1R, 2R)-2-aminocyclohexyl)-3-(((1R, 4aS, 10aR)-7-isopropyl-1, 4a-dimethyl-1, 2,3,4,4a,9,10,10a-octahydrophenanthren-1-yl)methyl)thiourea] structural formula is as follows:
[0047]
[0048] Dehydroabietamine-thiourea catalyst (1R, 2R)-CHDA-DHATU preparation method:
[0049] Dissolve 2.85g (10mmol) of dehydroabietamine in 50ml of anhydrous ether solution, and add 2.06g (10mmol) of dicyclohexylcarbodiimide (DCC). After stirring for 15 minutes, 4 ml (50 mmol) of carbon disulfide was added dropwise at 0° C., and the reaction was carried out at room temperature for 8 hours. After filtration and concentration of the filtrate, column chromatography (H60 silica gel, n-hexane:ethyl acetate=5:1) gave 3.1 g of isothiocyanate (95% yield).
[0050] Dissolve 1.14g (10mmol) (1R, 2R)-cyclohexanediamine in 40ml of anhydrous dichloromethane, and mix 3.1g of isothiocyanate di...
Embodiment 2
[0057] Embodiment 2: dehydroabietamine-thiourea catalyst (abbreviation: (1S, 2S)-CHDA-DHATU), [English full name: 1-((1S, 2S)-2-aminocyclohexyl)-3-(((1R , 4aS, 10aR)-7-isopropyl-1, 4a-dimethyl-1, 2,3,4,4a,9,10,10a-octahydrophenanthren-1-y1)methyl)thiourea] structural formula is as follows:
[0058]
[0059] The preparation method of dehydroabietamine-thiourea catalyst (1S, 2S)-CHDA-DHATU is the same as that of Example 1.
[0060] [α] 20 D =-57 (c=1.1, CHCl 3 ); melting point: 87-88C.
[0061] NMR analysis:
[0062] 1 H NMR (300MHz, [D 6 ]DMSO): δ7.40 (br, 1H), 7.14-7.16 (d, J=8.1Hz, 1H), 6.93-6.96 (dd, J=1.5Hz, 8.1Hz, 1H), 6.84 (s, 1H) , 3.75(br, 1H), 3.35-3.44(m, 2H), 2.71-2.78(m, 3H), 2.40-2.46(m, 1H), 2.24-2.28(d, J=12.9Hz, 1H), 2.00 (m, 1H), 1.47-1.87(m, 7H), 1.35-1.40(m, 2H), 1.21-1.25(m, 2H), 1.13-1.16(m, 9H), 0.95-0.96(m, 7H) ; 13 C NMR (75MHz, [D 6 ]DMSO): δ147.0, 144.9, 134.4, 126.4, 124.0, 123.5, 54.2, 44.5, 38.0, 37.4, 37.0, 34.2, 32.8, 31.5, 29.7, 25....
Embodiment 3
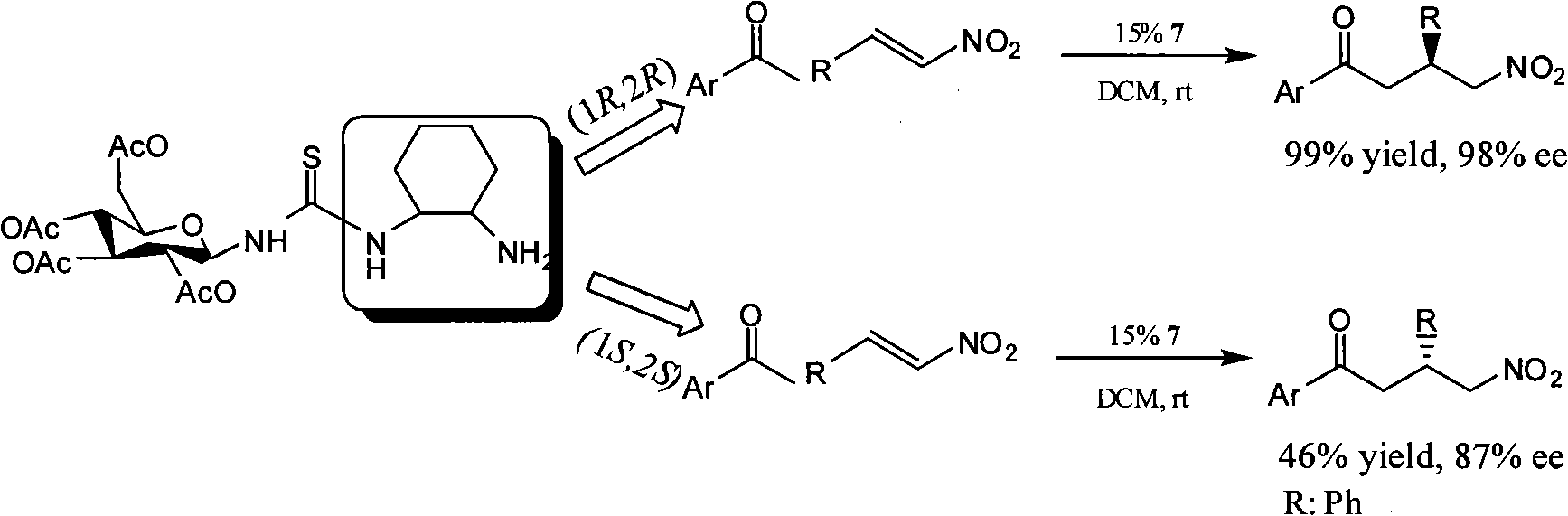
[0066] Example 3: (S)-4-nitro-1,3-diphenyl-1-butanone and (R)-4-nitro-1,3-diphenyl-1-butanone have the following structural formulas:
[0067]
[0068] a. The method for preparing (S)-4-nitro-1,3-diphenyl-1-butanone with dehydroabietamine-thiourea catalyst (1R, 2R)-CHDA-DHATU:
[0069] Dissolve 15mg (0.1mmol) substrate nitrostyrene, 6.6mg (0.015mmol) dehydroabietamine-thiourea catalyst (1R, 2R)-CHDA-DHATU, 1.8mg (0.015mmol) benzoic acid in 0.8ml di In methyl chloride, 35 μl (0.3 mmol) of acetophenone was added at 0° C., and after 2 hours of reaction, the reaction was continued at room temperature for 60 hours. After the reaction was complete, it was extracted three times with dichloromethane (8ml each time), and dried with 2g of anhydrous sodium sulfate. Concentration and column chromatography (H60 silica gel, petroleum ether: ethyl acetate = 8: 1) gave the final product (S)-4-nitro-1,3-diphenyl-1-butanone 26.6 mg (99 %Yield).
[0070] [α] 20 D =-22 (c=1.0, CHCl 3 ); ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com