Method for producing N- ethyl carbazole
A technology of ethyl carbazole and carbazole, applied in the field of synthesizing N-ethyl carbazole, can solve the problems of complex production process, high cost, long reaction time, etc., achieve high yield and content, low production cost, easy operation easy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
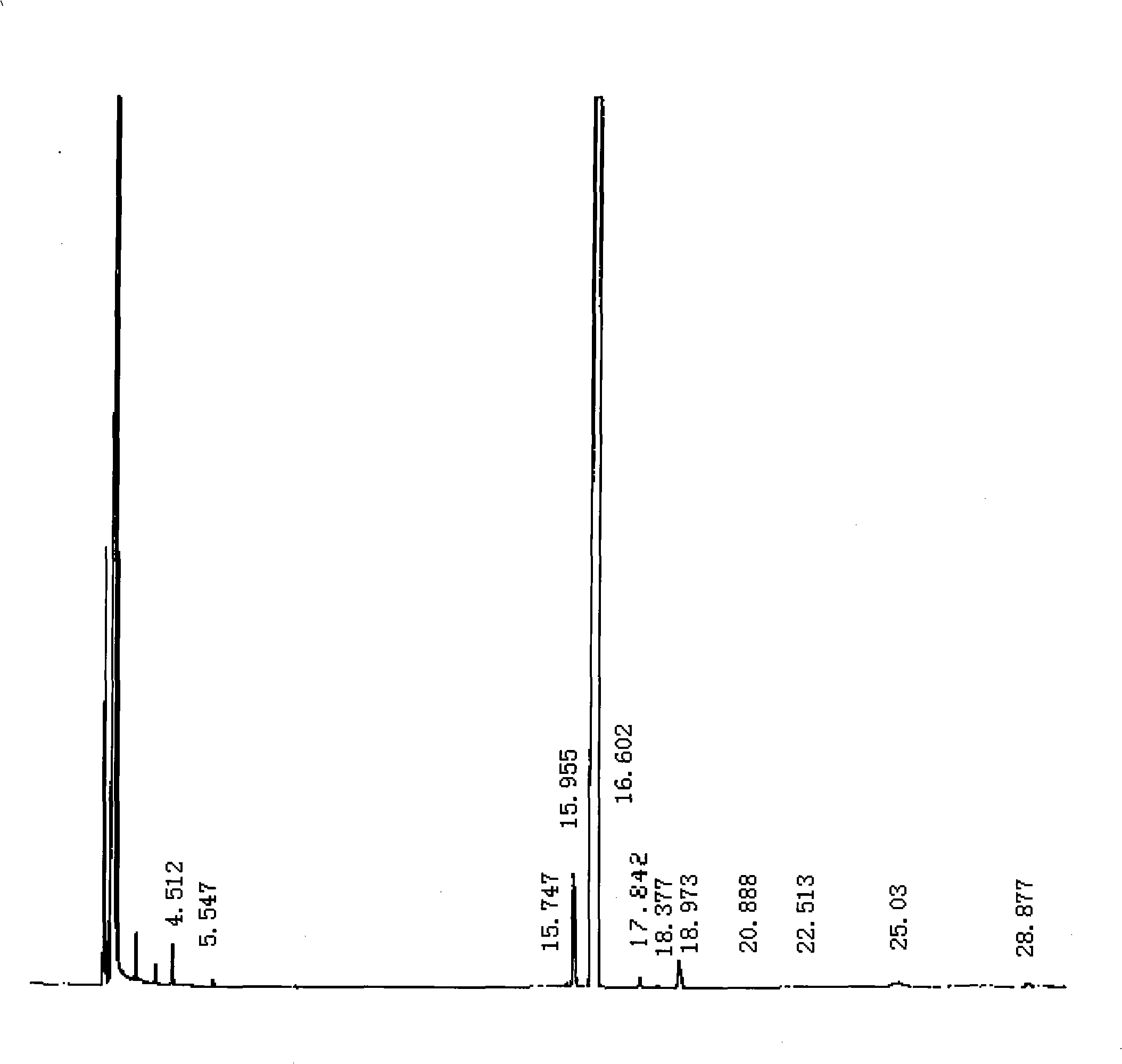
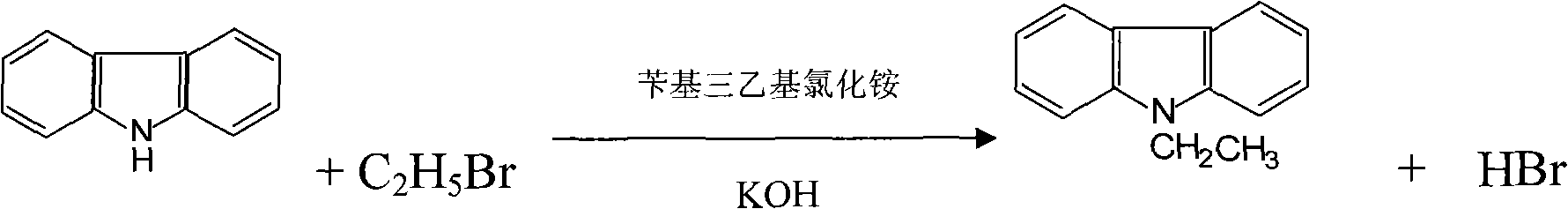
[0034] Put 300kg of toluene into the reaction kettle, add 100kg of 96% carbazole, 120kg of KOH and 1.5kg of benzyltriethylammonium chloride, start to stir and add 80kg of bromoethane dropwise for 45 minutes, and heat to 80°C Post-reaction for 5 hours. The mother liquor after the reaction was moved to a distillation pot, the solvent toluene was evaporated, and absolute ethanol was added for recrystallization, filtered and dried to obtain 99.74% N-ethylcarbazole.
[0035] The yield of N-ethylcarbazole was 98.3%.
[0036] After the KOH used after the reaction was washed with mixed xylenes, the above operation was repeated, and the yield of N-ethylcarbazole was 97.5%.
Embodiment 2
[0038]Add 8.3g carbazole, 0.1g catalyst benzyltriethylammonium chloride, 25g toluene, and 10g potassium hydroxide into a three-necked flask equipped with a reflux condenser, a thermometer, and a magnetic stirrer, heat in a water bath, and dropwise add Bromoethane 7g, dropwise for 30 minutes, heated to 75°C and reacted for 3h. The reaction was stopped, the solvent toluene in the reaction mother liquor was evaporated, methanol was added to recrystallize the product, and 99.86% N-ethylcarbazole was obtained by filtration.
[0039] The yield of N-ethylcarbazole was 98.1%.
[0040] After the KOH used after the reaction was washed with mixed xylenes, the above operation was repeated, and the yield of N-ethylcarbazole was 97.7%.
Embodiment 3
[0042] Put 400kg of toluene into the reaction kettle, add 100kg of 97% carbazole, 150kg of KOH and 2kg of benzyltriethylammonium chloride, start to stir and add 85kg of bromoethane dropwise for 45 minutes, after heating to 85°C React for 4 hours. Move the reacted mother liquor to a distillation pot, evaporate the solvent toluene, add 95% ethanol solution for recrystallization, filter and dry to obtain 99.68% N-ethylcarbazole.
[0043] The yield of N-ethylcarbazole was 98.8%.
[0044] After the KOH used after the reaction was washed with mixed xylenes, the above operation was repeated, and the yield of N-ethylcarbazole was 98.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com