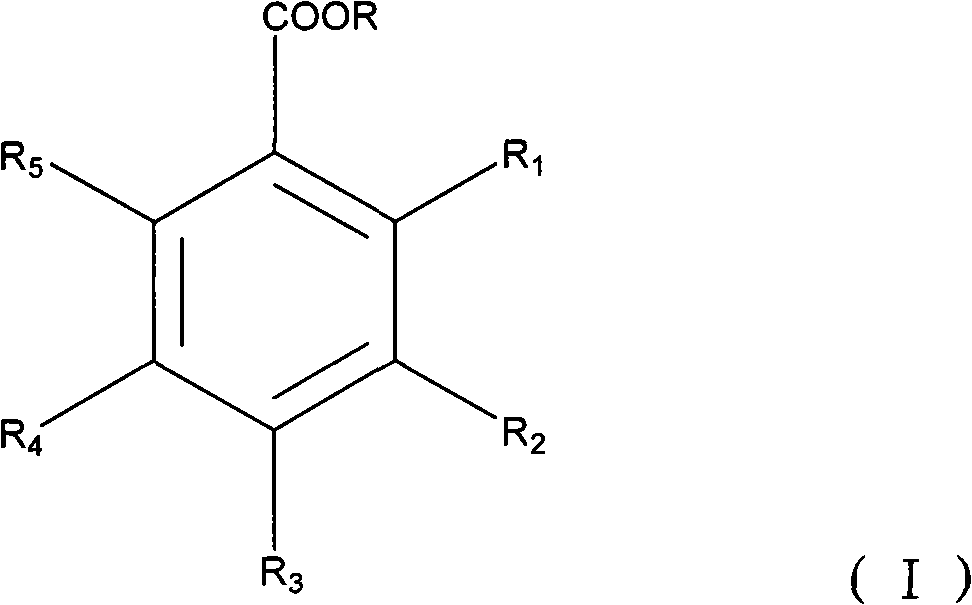
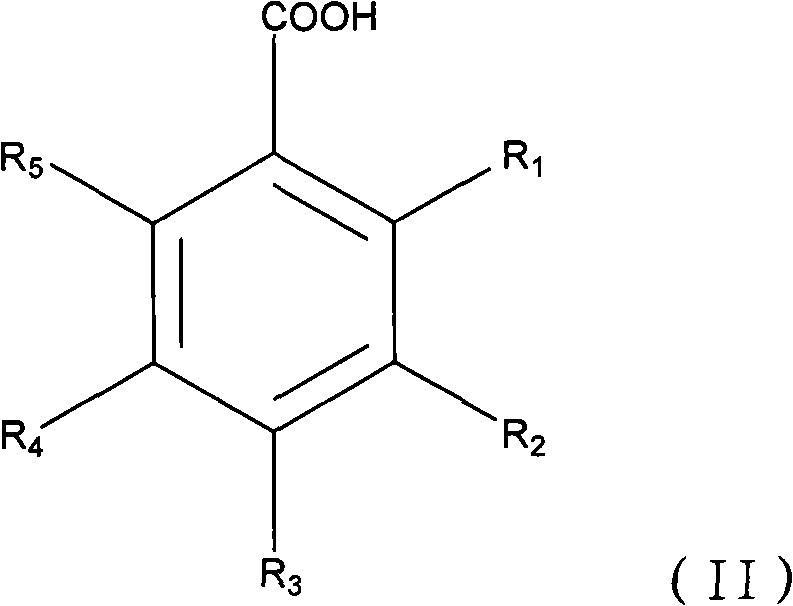
Application of hydroxyl benzoate and analogue in preparing medicament for preventing and treating hepatitis B virus
A technology of hydroxybenzoate and hepatitis B virus, which is applied to the application field of hydroxybenzoate and its analogs in the preparation of medicines for preventing and treating hepatitis B virus, and can solve temporary effects and large toxic reactions. , Zidovudine due to poor efficacy and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1 Preparation of anti-HPV virus microemulsion
[0049] Mix 20 ml of 1,2-propanediol, 8015 ml of Tween, and 5 ml of azone, and add sterile distilled water to a total volume of 100 ml to obtain an external preparation solution. Take benzoic acid, salicylic acid, benzoic acid + salicylic acid, p-hydroxybenzoic acid, 2,3-dihydroxybenzoic acid, 2,4-dihydroxybenzoic acid, 3,4-dihydroxybenzoic acid, p-hydroxybenzene Ethyl formate, methyl 3,4-dihydroxybenzoate, gallic acid, lauryl gallate, octyl gallate, propyl gallate, ethyl gallate, methyl gallate, 2,4-di Each 10g of methyl hydroxybenzoate was added with 50ml of external preparation solution to adjust the pH to 5.5, and then the external preparation solution was added to 100ml to obtain 10% anti-HPV virus microemulsion of the present invention and control microemulsion.
Embodiment 2
[0050] Example 2 Preparation of anti-HBV virus propyl gallate injection
[0051] Take 10g of propyl gallate, 8.5g of sodium chloride, 10ml of 1,2-propanediol, and 8010ml of Tween. After mixing, add sterile distilled water to dissolve, and add sterile distilled water to 100ml, adjust the pH to 7.4, and filter. Potting and sterilizing at 100°C for 30 minutes. Get Propyl Gallate Injection.
Embodiment 3
[0052] Example 3 Preparation of compound ethyl gallate effervescent tablets
[0053] Take 0.25 g of ethyl gallate, 0.01 g of polyinositides, and 0.45 g of tartaric acid, respectively, and pass through an 80-mesh sieve, and use 95% ethanol to make a soft material, pass through a 12-mesh sieve to make wet granules, and dry at 50° C. for use. Take another 0.65g of sodium bicarbonate, 0.02g of dextrin and sterile distilled water to make soft material, pass through a 12-mesh sieve to make wet granules, dry at 50℃, then mix with the above dry granules, sizing, add appropriate amount of sterilized distilled water, and bake After a while, add 0.01g PEG6000. Mix well, press tablets, and get ready.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com