Catalyst for carbon dioxide dry-reforming of methane, and preparation method and use thereof
A technology of catalysts and oxides, applied in the direction of metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, chemical instruments and methods, etc., can solve complex preparation processes, poor stability, and easy carbon deposition of catalysts, etc. problem, to achieve the effects of simple preparation process, strong anti-sintering, and strong anti-surface carbon deposition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
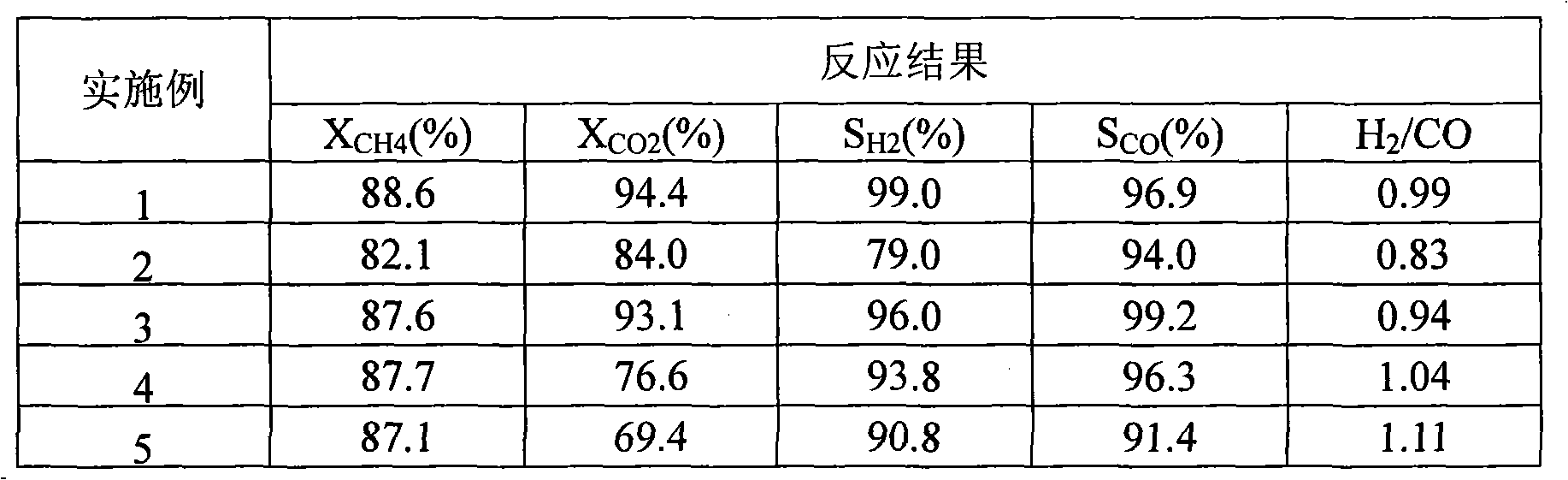
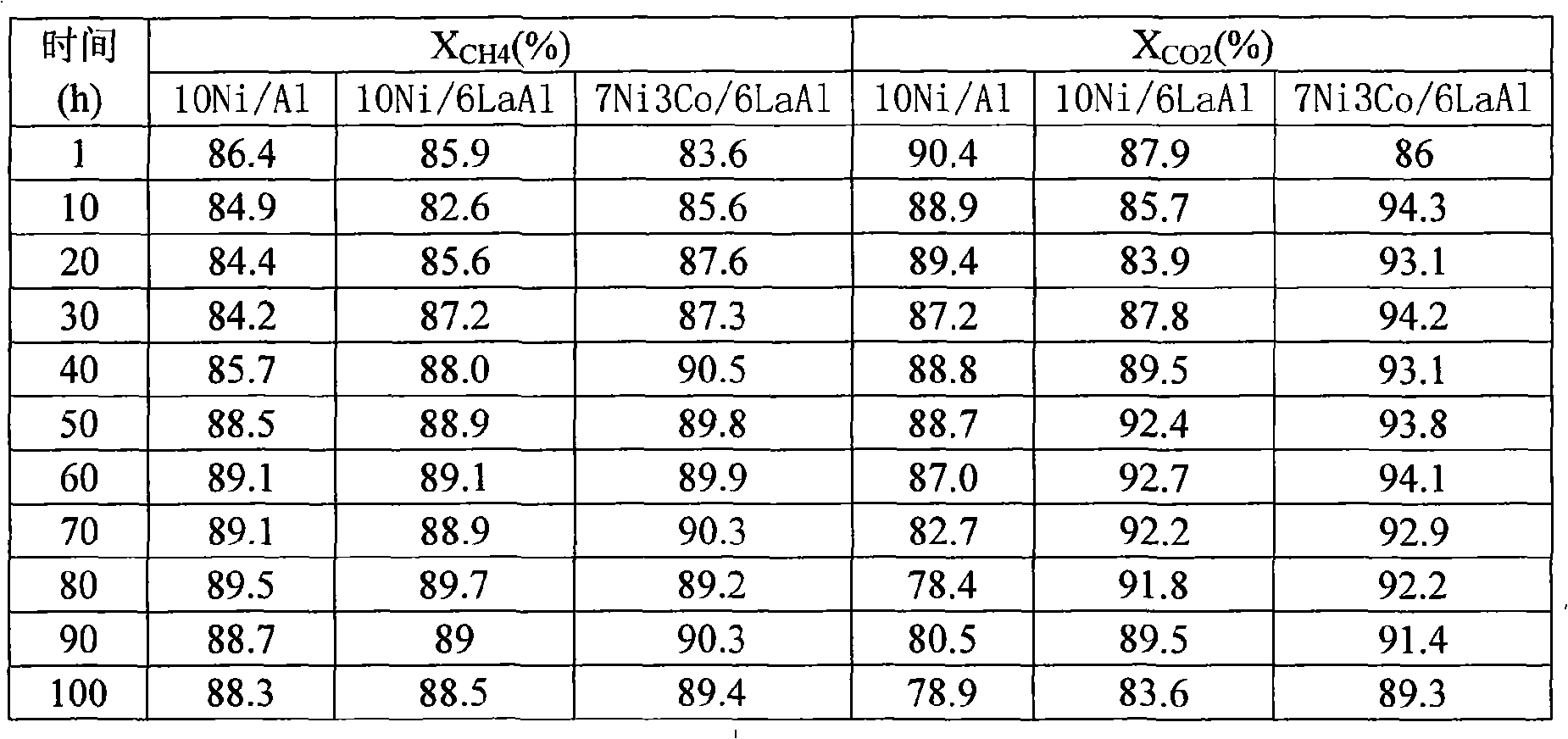
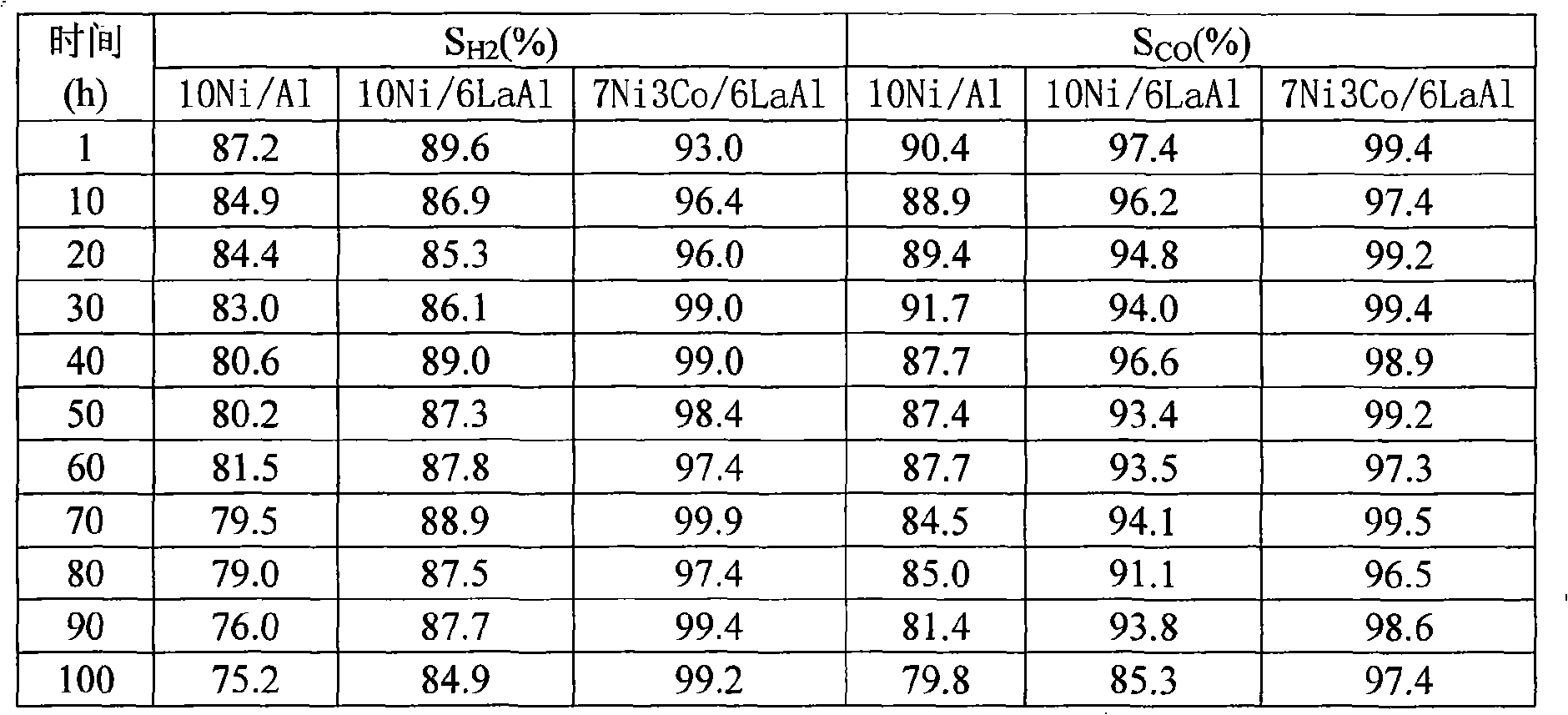
Examples
Embodiment 1
[0016] Example 1 (3Ni7Co6La 2 o 3 84Al 2 o 3 ):
[0017] Weigh 0.6818g of lanthanum nitrate and dissolve it in 10ml of deionized water to make a solution, then weigh 4.2g of γ-Al 2 o 3 , uniformly impregnate the above-mentioned lanthanum nitrate solution into the powder carrier γ-Al 2 o 3 , standing at room temperature for 6 hours, drying at 120°C for 5 hours, and then calcining at 550°C for 6 hours in an air atmosphere to obtain a modified catalyst carrier. Weigh nickel nitrate, cobalt nitrate 0.7432g, 1.7342g and dissolve in 10ml deionized water to prepare mixed nitrate solution, impregnate evenly on the above-mentioned modified catalyst carrier, let stand at room temperature for 6 hours, and dry at 150°C for 5 hours, and then calcined at 750°C for 6 hours in an air atmosphere to obtain a sample catalyst.
Embodiment 2
[0018] Embodiment 2 (5Ni5Co6La 2 o 3 84Al 2 o 3 ):
[0019] Weigh nickel nitrate, cobalt nitrate, lanthanum nitrate 1.2387g, 1.2387g, 0.6818g respectively, and the rest of the steps are the same as in Example 1.
Embodiment 3
[0020] Embodiment 3 (7Ni3Co6La 2 o 3 84Al 2 o 3 ):
[0021] Weigh nickel nitrate, cobalt nitrate, lanthanum nitrate 1.7342g, 0.7432g, 0.6818g respectively, and the remaining steps are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com