Spirulina phycobiliprotein alpha subunit total protein gene and encoding protein thereof
A technology of phycobiliprotein and spirulina, which is applied in the field of genetic engineering, can solve the problems that ordinary consumers cannot afford, hinder market expansion, and cumbersome steps, so as to promote high and stable production, have obvious ecological and environmental benefits, and avoid the limitation of raw materials Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
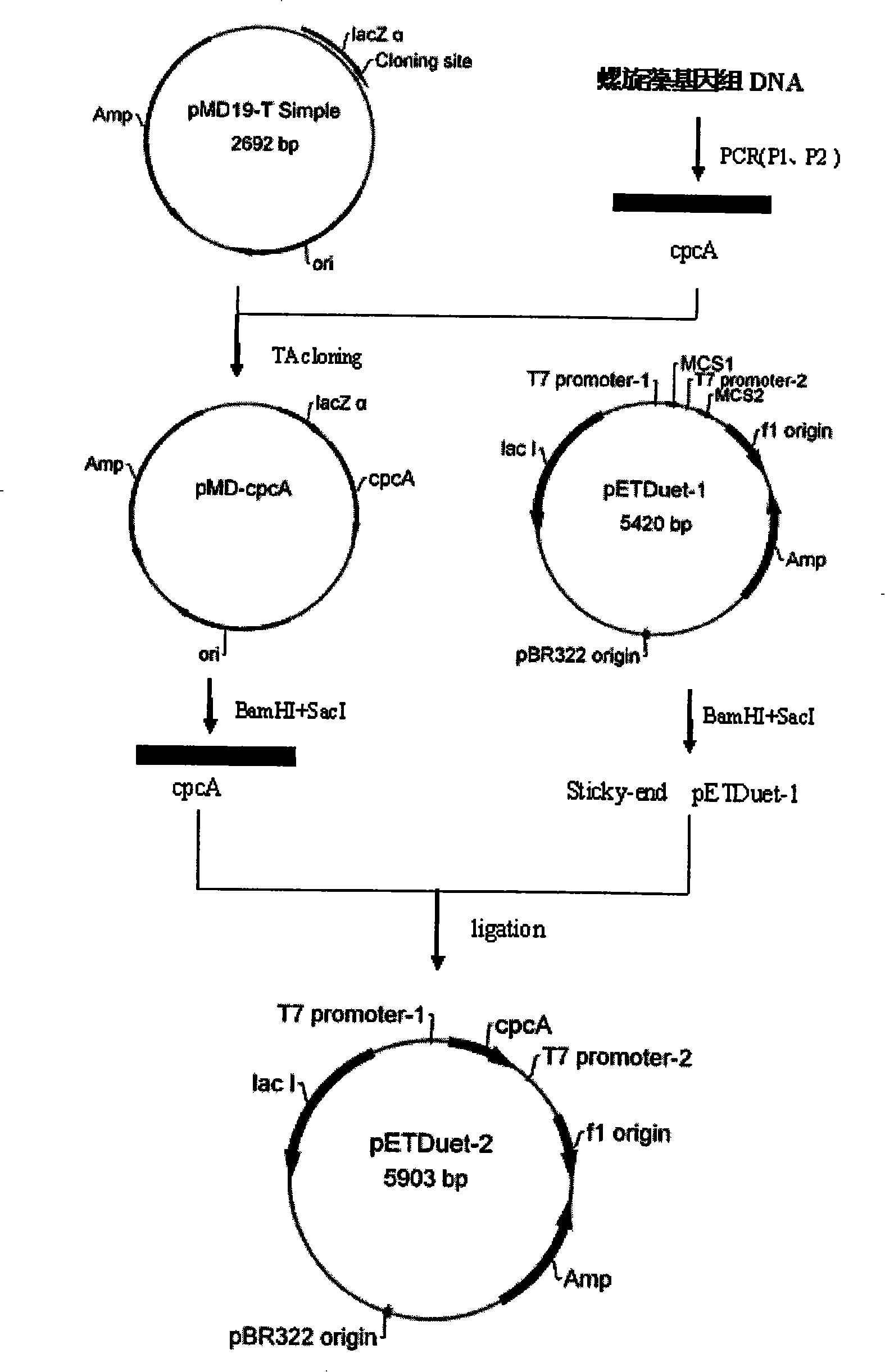
[0015] Example 1. Obtaining of Spirulina Phycobiliprotein α Submatrix Plasmid
[0016] The extraction method of Spirulina genomic DNA is as follows: Take 200mg of fresh algae mud into a 5ml sterilized centrifuge tube, freeze and thaw with liquid nitrogen for 2 to 3 times, then add 1.5ml of CTAB extraction buffer [1.5%CTAB] preheated to 65°C , 100mmol / L Tris-HCl (pH 8.0), 1.4mol / L NaCl, 20mmol / LEDTA (pH 8.0), 1% (V / V) β-mercaptoethanol (added before use)], add proteinase K solution to the final The concentration was 200 μg / ml. After suspending and mixing evenly, bathe in water at 65°C for 1 hour, and mix by gently inverting from time to time. Then centrifuge at 10000r / min for 5min to remove impurities such as cell debris, add 1.5ml chloroform / isoamyl alcohol (24:1) to the supernatant, mix well and centrifuge at 12000r / min for 10min at room temperature. The supernatant was added with 150 μl of 3 mol / L NaAc (pH 5.2) and 3 ml of pre-cooled absolute ethanol, placed at -20°C for 1...
Embodiment 2
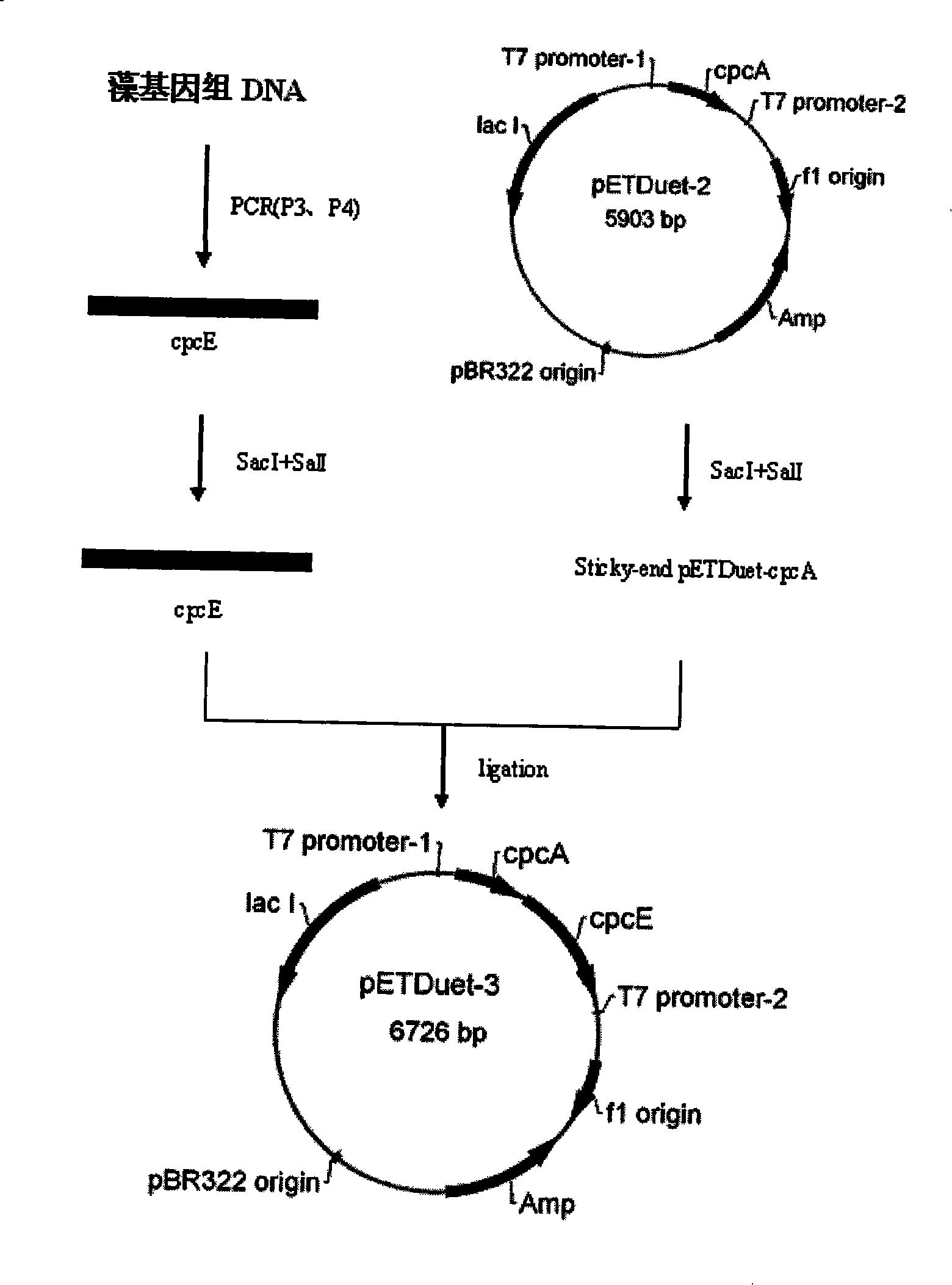
[0022] Example 2. Construction of Spirulina phycobiliprotein alpha subunit, lyase E expression vector
[0023] Using genomic DNA as a template, the lyase E gene was amplified by PCR. The primer sequences are as follows:
[0024] P3: 5′-CCGAGCTCATGAGTGAACCTAACC-3′
[0025] P4: 5′-CCGTCGACTCAGAGTAAACTATCC-3′
[0026] After the amplified fragment was digested by SacI and SalI, the gene was connected to pETDuet-2 which had been digested by the same double enzymes with Solution I to obtain the expression vector pETDuet-3. See the build process figure 2 .
Embodiment 3
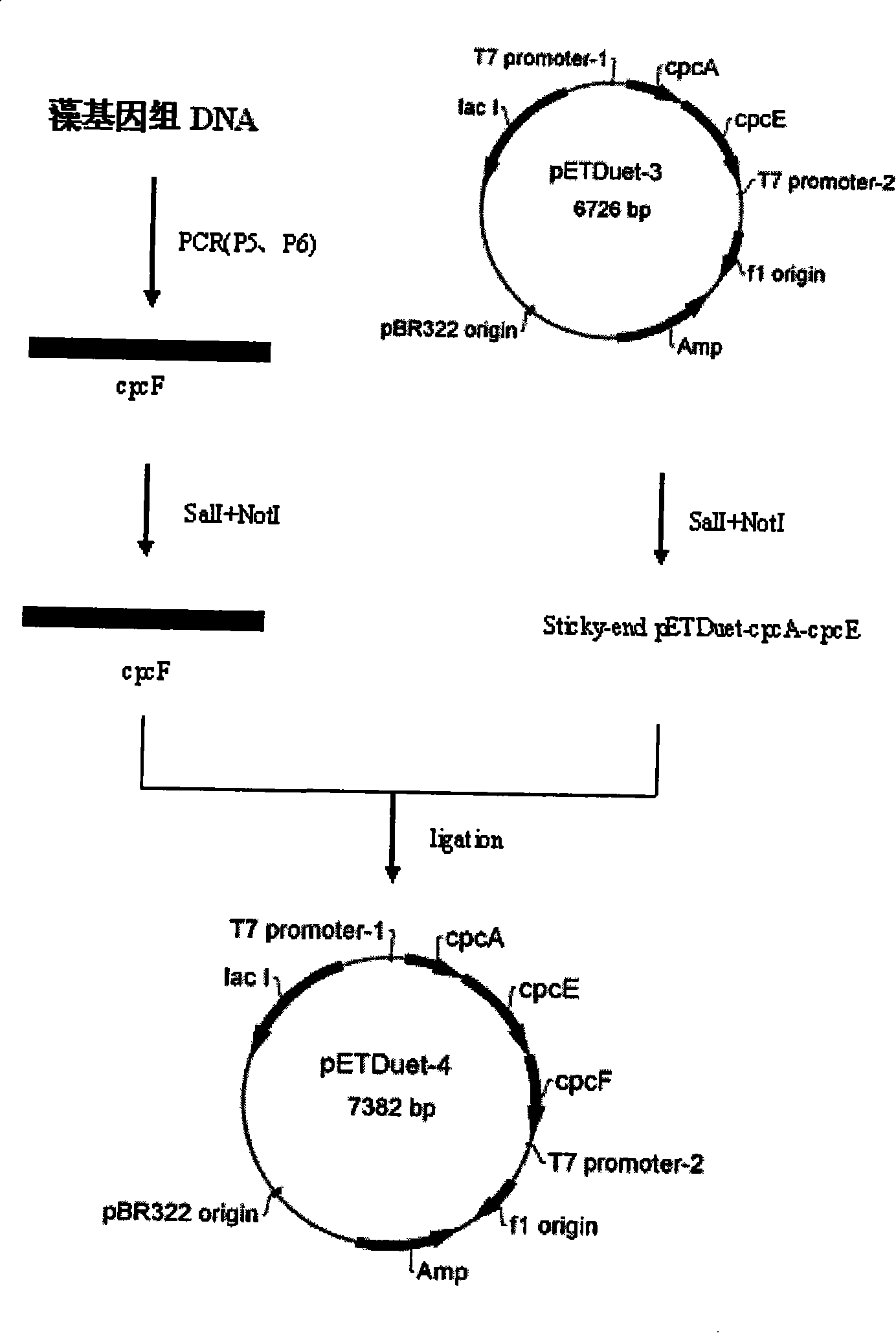
[0027] Example 3. Construction of Spirulina phycobiliprotein alpha subunit, lyase E / F expression vector
[0028] Using genomic DNA as a template, the lyase F gene was amplified by PCR. The primer sequences are as follows:
[0029] P5: 5′-CCGTCGACATGGAGGGTAATAGCGTC-3′
[0030] P6: 5′-TATGCGGCCGCCTAGATTGGGCCGATGTTTTCCAGG-3′
[0031] After the amplified fragment was digested by SalI and NotI, the gene was connected to pETDuet-3 which had been digested by the same double enzymes with Solution I to obtain the expression vector pETDuet-4. See the build process image 3 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com