Cathode material for solid-oxide fuel cell and method for preparing the same
A solid oxide and cathode material technology, which is applied to fuel cell components, battery electrodes, circuits, etc., can solve the problems of high operating temperature of SOFC, sintering of precious metal particles, and increase of battery interface polarization resistance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] La 0.76 Sr 0.19 Ag 0.05 MnO 3-δ Preparation and application of cathode materials.
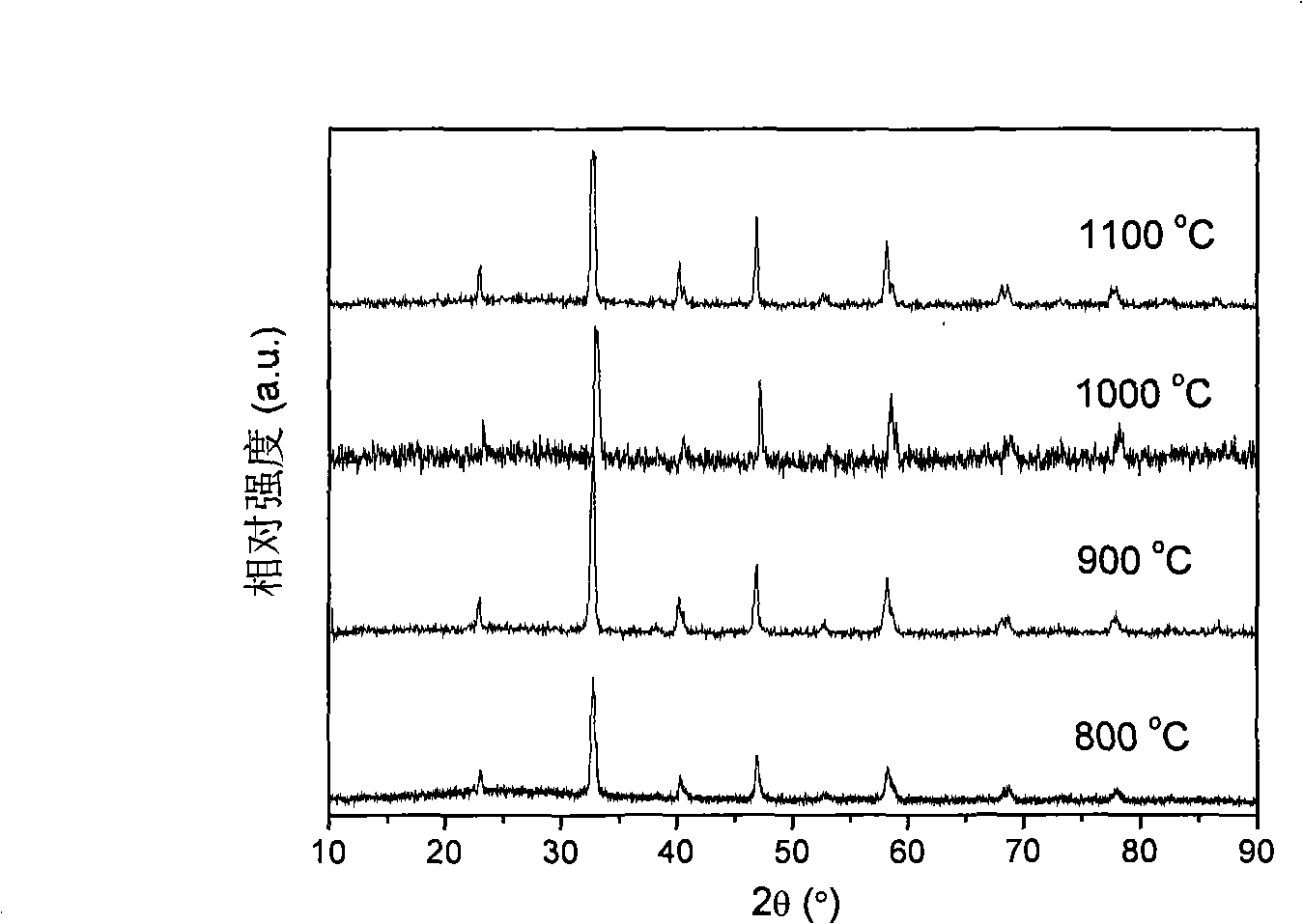
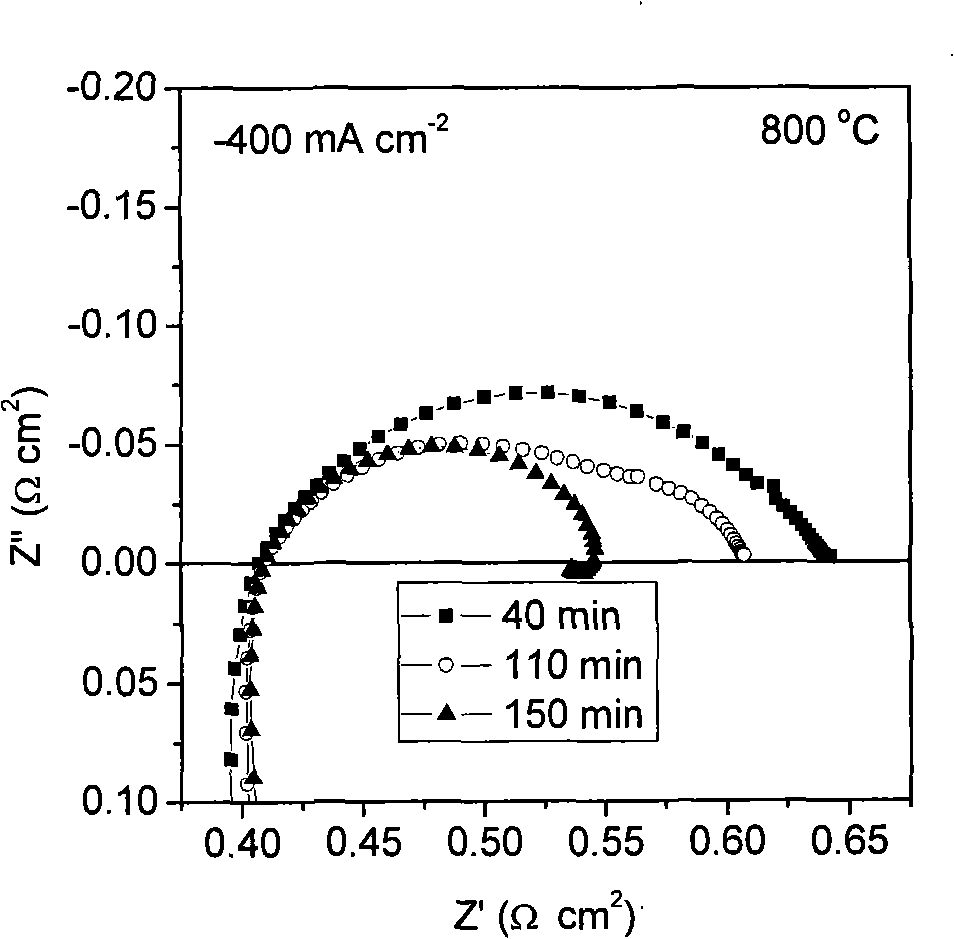
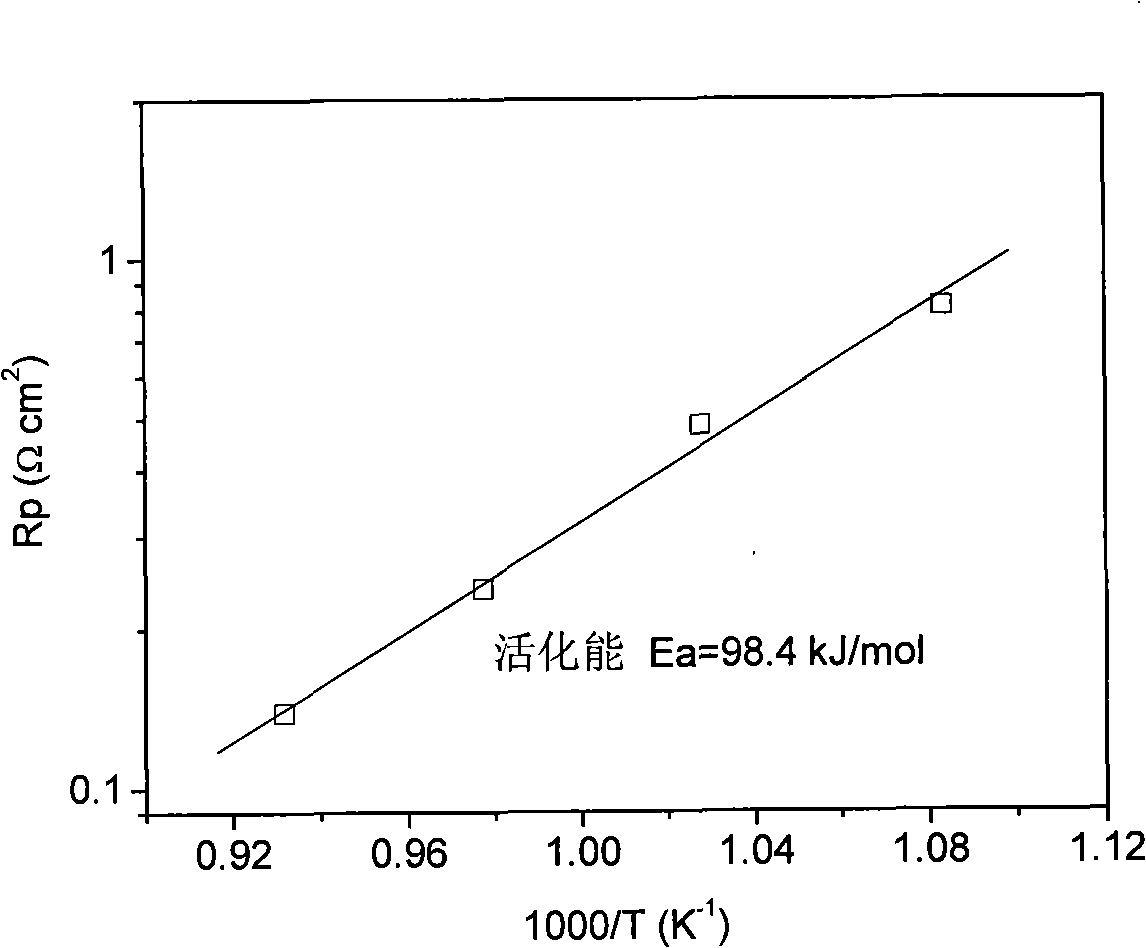
[0030] La 0.76 Sr 0.19 Ag 0.05 MnO 3-δ It is synthesized by EDTA-citric acid joint complexation method, which belongs to sol-gel method. Sr(NO 3 ) 2 , La(NO 3 ) 3 , AgNO 3 and Mn(CH 3 COO) 2 Dissolve in deionized water in proportion to make a mixed solution, according to the molar ratio of metal ion: EDTA: citric acid: ammonia water is 0.1: 0.1: 0.2: 1, add an appropriate amount of EDTA-ammonia solution and citric acid, stir and clarify the The precursor solution was evaporated on a heating platform at 80°C to remove water to make it into a gel, and then placed in a blast drying oven at 250°C for 8 hours to obtain a solid precursor, which was calcined at a high temperature for 5 hours to obtain La 0.76 Sr 0.19 Ag 0.05 MnO 3-δ Cathode powder. figure 1 It is the X-ray diffraction patterns of the cathode material after firing at different temperatures. The results prove ...
Embodiment 2
[0035] LaCo 0.38 Fe 0.57 PD 0.05 o 3-δ Preparation and application of cathode materials.
[0036] LaCo 0.38 Fe 0.57 PD 0.05 o 3-δ It is synthesized by glycine combustion method, which belongs to self-combustion method. La(NO 3 ) 3 , Co(NO 3 ) 2 and PdCl 2 Dissolve in deionized water in proportion to make a mixed solution, add an appropriate amount of glycine according to the molar ratio of metal ion: glycine is 1:2, and evaporate the precursor solution after stirring and clarification on a heating platform at 80°C to remove water and make it into a solidified solution. Colloidal, then placed in a blast drying oven at 250 ° C to trigger spontaneous combustion to obtain LaCo 0.38 Fe 0.57 PD 0.05 o 3-δ Cathode powder. Figure 6 It is the X-ray diffraction pattern of the cathode material prepared after calcination at 800°C for 5 hours. The results prove that the cathode material can be formed after self-combustion.
[0037] 5 g LaCo was processed by high-energy ...
Embodiment 3
[0040] La 0.4 Ca 0.4 Ag 0.15 PD 0.05 V 0.3 co 0.7 o 3-δ Preparation of cathode materials.
[0041] La 0.4 Ca 0.4 Ag 0.15 PD 0.05 V 0.3 co 0.7 o 3-δ It is synthesized by EDTA-citric acid joint complexation method, which belongs to sol-gel method. La(NO 3 ) 3 , Ca(NO 3 ) 2 , AgNO 3 , Co(NO 3 ) 2 , PdCl 2 and VCl 3 Dissolve in deionized water in proportion to make a mixed solution, add an appropriate amount of EDTA-ammonia solution and citric acid according to the molar ratio of metal ion: EDTA: citric acid: ammonia water is 0.05: 0.05: 0.1: 1, stir and clarify the The precursor solution was evaporated on a heating platform at 100°C to remove water to make it into a gel, and then placed in a blast drying oven at 300°C for 5 hours to obtain a solid precursor, which was calcined at a high temperature for 8 hours to obtain La 0.4 Ca 0.4 Ag 0.15 PD 0.05 V 0.3 co 0.7 o 3-δ Cathode powder.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com