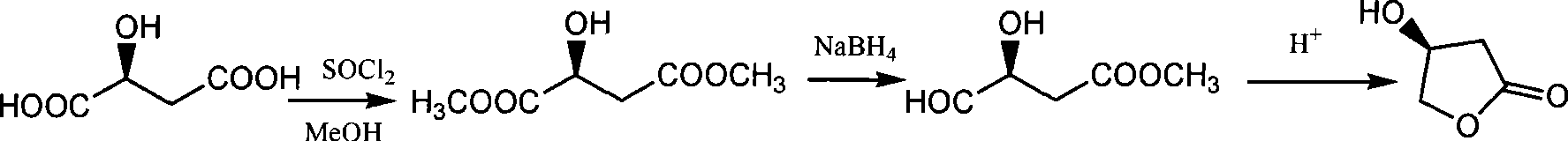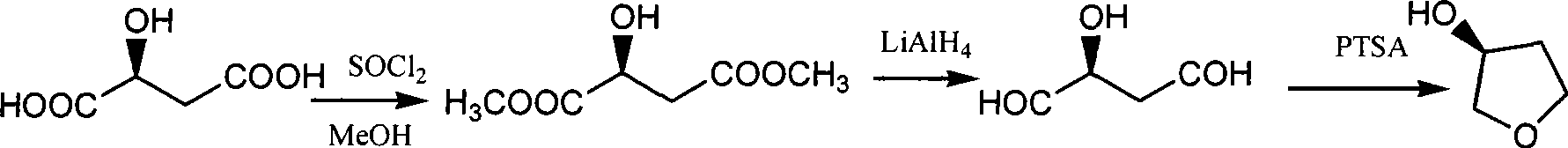Joint production method for (S)-3-hydroxyl-gamma-butyrolactone, (S)-3-hydroxyl tetrahydrofuran
A hydroxytetrahydrofuran, combined production technology, applied in the directions of organic chemistry methods, chemical instruments and methods, asymmetric synthesis, etc., can solve the problems of low lactone content, low optical purity, difficulty in separating lactones, etc., and reduce production costs. , the effect of reducing dosage and reducing pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] To prepare L-dimethyl malate, dissolve 50 g of L-malic acid (0.373 mol) in 210 ml of methanol, add 3 g of concentrated sulfuric acid, heat and reflux for 3 hours, evaporate the methanol to dryness under reduced pressure, then add 160 ml of methanol and 10 ml of dichloromethane Sulfone was heated and refluxed for 2 hours, adjusted to pH = 6.5 with aqueous sodium hydroxide solution, filtered and evaporated to dryness of methanol under reduced pressure to obtain 59.7 g of light yellow liquid with a yield of 98.7%.
Embodiment 2
[0028] To prepare L-diethyl malate, dissolve 50 g of L-malic acid (0.373 mol) in 250 ml of ethanol, add 3 g of concentrated sulfuric acid, heat and reflux for 3 hours, evaporate the solvent to dryness under reduced pressure, then add 180 ml of ethanol and 12 ml of dichlorohydrin Sulfone was heated and refluxed for 2 hours, adjusted to pH=6.5 with aqueous sodium hydroxide solution, filtered and evaporated to dryness of ethanol under reduced pressure to obtain 68.9 g of light yellow liquid with a yield of 97.3%.
Embodiment 3
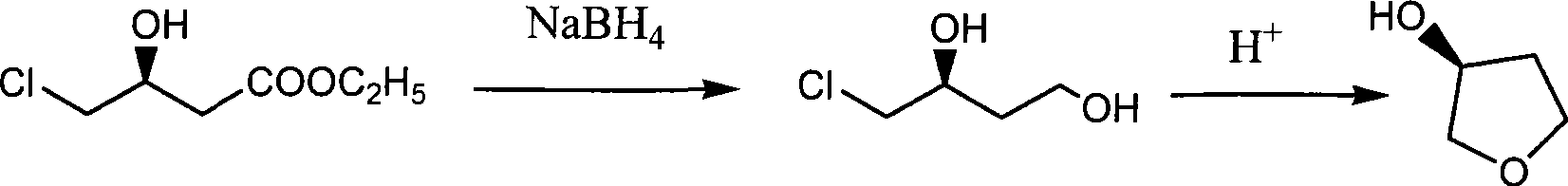
[0030] To prepare (S)-3-hydroxy-γ-butyrolactone and (S)-3-hydroxytetrahydrofuran, add 43.79g (0.32mol) of anhydrous zinc chloride and 25.1g (0.46mol) of potassium borohydride to 450ml of tetrahydrofuran , 50g (0.31mol) L-dimethyl malate, stirring at 15°C to carry out the reduction reaction, after the TLC detection of the raw material reaction, evaporate the solvent, add 1000ml of water and stir, filter to remove the solid, and the filtrate is electrodialyzed to obtain (S )-1,2,4-butanetriol aqueous solution and (S)-3,4-dihydroxybutyrate sodium aqueous solution;
[0031] Distill (S)-1,2,4-butanetriol aqueous solution to remove water to obtain colorless viscous liquid (S)-1,2,4-butanetriol, add p-toluenesulfonic acid 0.5g (2.9mmol) , heated to 175°C, rectified under reduced pressure to obtain 9.8g (S)-3-hydroxytetrahydrofuran, yield 36.2%;
[0032] Adjust the aqueous solution of (S)-3,4-dihydroxybutyrate sodium to pH 1.0 with aqueous hydrochloric acid, distill off the water aft...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com