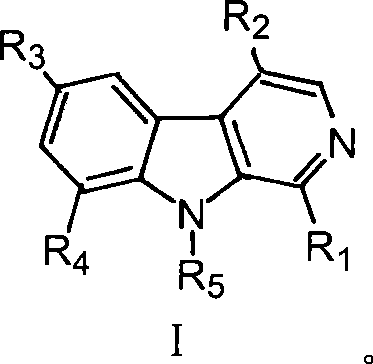
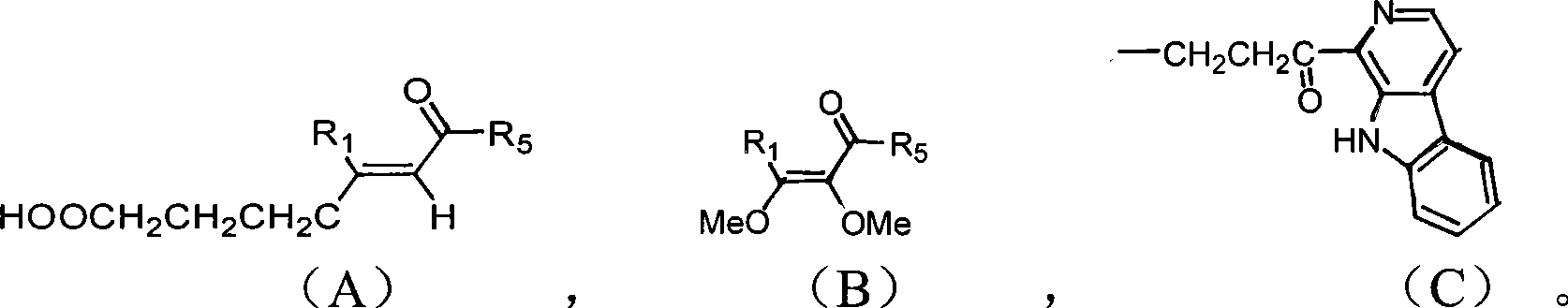
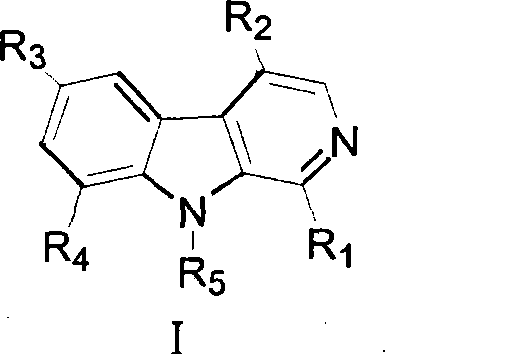
Beta-kabarin alkaloids in quassia wood, preparation method and application thereof
A technology of carbaline and alkaloid, applied in the field of traditional Chinese medicine, can solve the problem that the anti-inflammatory effect has not been reported yet, and achieve the effect of good research and development prospect and novel structure.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1: Extraction and separation of alkaloids in bitter wood
[0038] Take 10kg of dried root of Picrasma quassioides, extract under reflux with 60% ethanol, concentrate the extract to dryness, obtain 100g of dry extract, extract 3 times with equal volume of chloroform, and extract with chloroform part, concentrated to dryness under reduced pressure, and weighed 64g, the chloroform extraction part was eluted with cyclohexane-ethyl acetate (50:50-0:100) gradient to obtain 10 fractions PQC1 to PQC10, PQC7 (cyclo Hexane-ethyl acetate 30:70, 18.1g) was subjected to ODS medium and low pressure column chromatography to obtain 9 subfractions PQC7-1 to PQC7-9. Using preparative liquid phase, alkaloids 1-6 were isolated from sub-fraction PQC7-6 (methanol-water 50:50, 650 mg), and alkaloids 1-6 were isolated from sub-fraction PQC7-8 (methanol-water 60:40, 845 mg). Bases 7 and 8, alkaloids 9, 10 and 11 were isolated from subfraction PQC7-9 (methanol-water 70:30, 920 mg). ...
Embodiment 2
[0067] Example 2: Inhibitory activity experiment of bitterwood alkaloids 1-11 on release of nitric oxide (NO) from mouse mononuclear macrophage RAW 264.7 induced by lipopolysaccharide
[0068] Mouse mononuclear macrophage RAW 264.7 (ATCC TIB-71) was cultured in 10% heat-inactivated (56°C, 30min) fetal bovine serum (FBS), 100 U / mL penicillin sodium (Gibco), 100 μg / mL streptavidin in RPMI 1640 (Gibco) medium, 37°C, 5% CO 2 grown in a constant temperature incubator.
[0069] Because NO is extremely unstable, it is quickly metabolized into nitrous acid (NO) in the cell culture supernatant. 2 - ), so the Griess method was used to measure NO in the sample 2 - concentration as an indicator of NO levels. Griess reagent A: 0.1% N-naphthalene diamine dihydrochloride dissolved in water; Griess reagent B: 1% p-sulfanilamide dissolved in 5% H 3 PO 4 middle. Mix reagents A and B in equal volumes before use.
[0070] Dilute RAW 264.7 cells to 5×10 with RPMI 1640 medium 5 cells / mL c...
Embodiment 3
[0073] Example 3: Inhibitory activity experiment of bitterwood alkaloids 1-11 on lipopolysaccharide-induced release of tumor necrosis factor-α (TNF-α) from mouse mononuclear macrophage RAW264.7
[0074] Cell culture is the same as NO release inhibitory activity test.
[0075] The inhibitory activity of TNF-α was tested using the mouse TNF-α ELISA kit (R&D).
[0076] Dilute RAW 264.7 cells to 5×10 with RPMI 1640 medium 5cells / mL concentration, seeded in a 96-well cell culture plate, and added 200 μL of cell suspension to each well. CO 2 After culturing in the incubator for 1 h, add lipopolysaccharide (lipopolysaccharide, LPS) (Sigma) (final concentration 1 μg / mL) and 0.4 μL of different concentrations of test samples dissolved in DMSO to each well, and set up the LPS group at the same time (add LPS, but do not add Test sample, the inhibition rate to TNF-α release is 0%) and blank control group (do not add LPS and test sample, only add 0.4 μ L DMSO, the inhibition rate to TNF...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com