Method for synthesis of high purity trimethyl silicon based imidazole
A technology of trimethylsilyl imidazole and a synthesis method, which is applied in the directions of silicon organic compounds, sustainable manufacturing/processing, climate sustainability, etc. It can reduce the production of imidazole and silyl ether, shorten the reaction time, and simplify the process flow.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Add 34g (0.5mol) imidazole and 1.2g (0.002mol) magnesium stearate in a 250 milliliter three-necked flask with constant pressure dropping funnel, thermometer, magnetic stirring and 1 meter rectifying column, and 96.6g (0.6 mol) hexamethyldisilazane was added to the constant pressure dropping funnel. Then heat, turn on magnetic stirring, control the temperature of the reaction kettle at about 140°C, slowly add hexamethyldisilazane dropwise from the constant pressure funnel, and rectify while reacting, the produced by-product ammonia gas is absorbed by water, and the rectification column The fraction below 105°C was collected at the top, and the reaction time was controlled to be 2 hours. After the reaction, the reaction solution was rectified under reduced pressure to obtain a high-content trimethylsilyl imidazole product with a yield of 88% and a content of 99.3%.
Embodiment 3-8
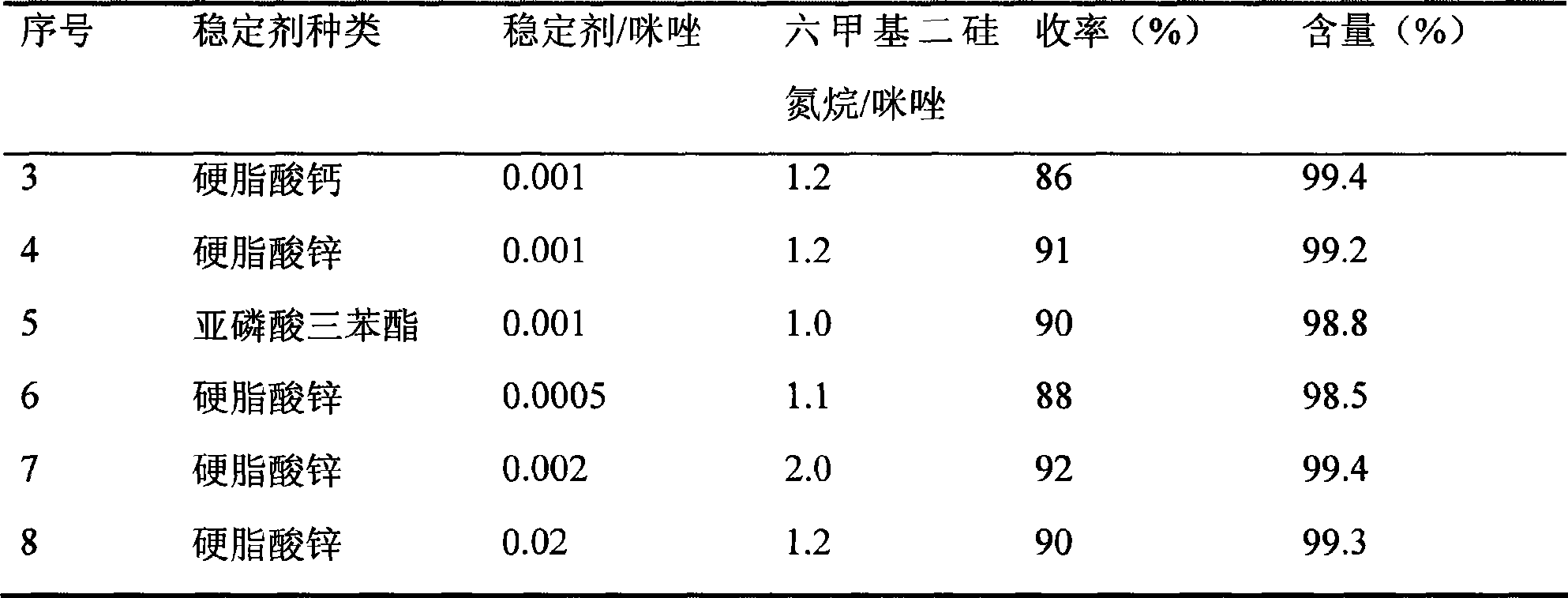
[0018] Similar to Example 1, the control reaction temperature is 140 DEG C, and the reaction time is 2 hours, and the type of stabilizer and the amount of catalyst are changed, and the reaction results are as follows (Table 1):
[0019] Table 1 Effects of different stabilizer types and amounts on the reaction
[0020]
Embodiment 9-15
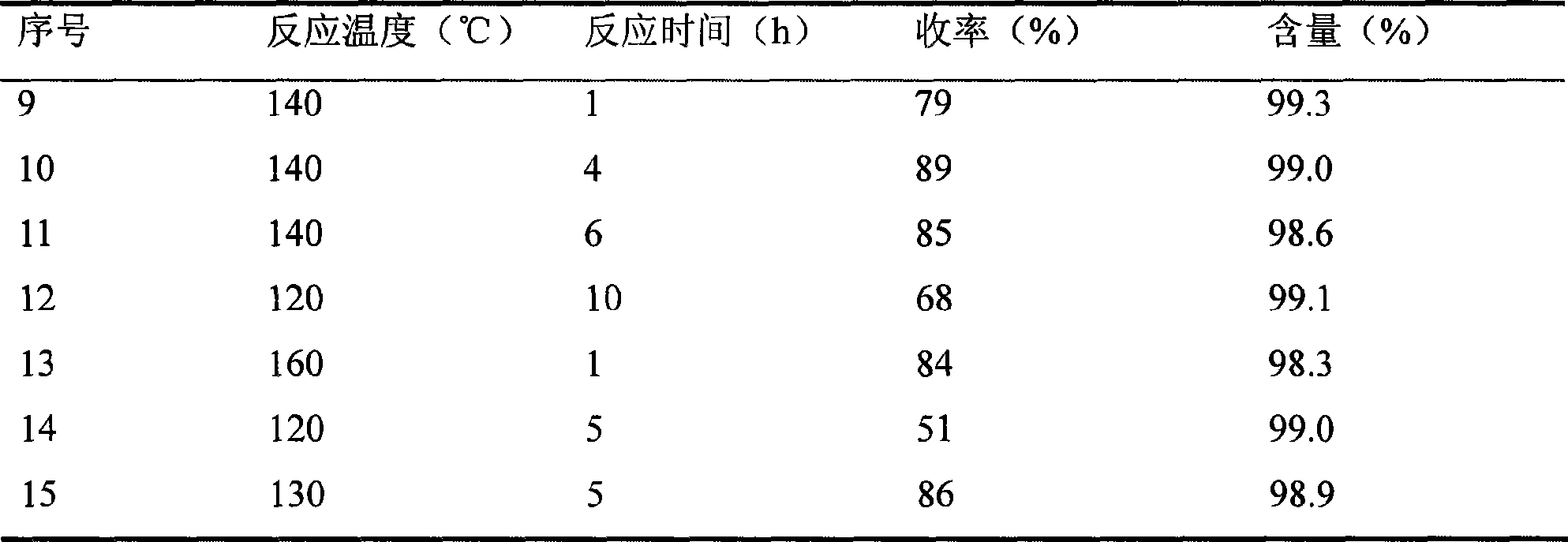
[0022] Similar to Example 1, using zinc stearate as a stabilizer, the molar ratio of zinc stearate and imidazole is 0.001: 1, the molar ratio of hexamethyldisilazane and imidazole is 1.2: 1, change the reaction temperature, The condition of reaction pressure and reaction time, reaction result is as follows table (table two):
[0023] Table 2 The influence of different reaction conditions on the reaction
[0024]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com