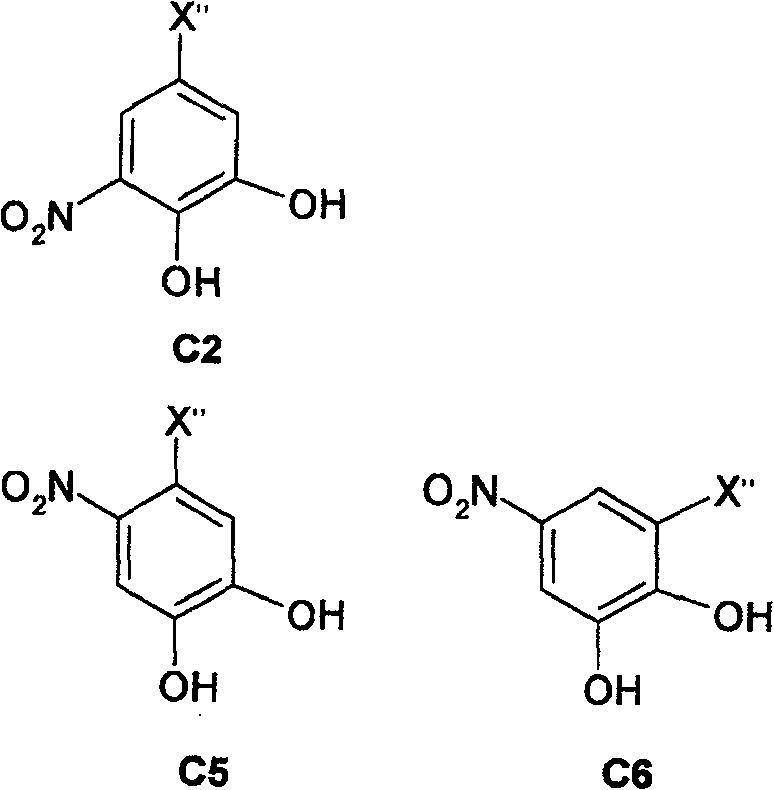
Novel nitrocatechol derivatives having selectin ligand activity
A technology for selecting proteins and drugs, applied in the field of compounds for in vitro and in vivo processes, capable of solving problems such as low potency and high molecular weight
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment A
[0215] Preparation of [5-(2-amino-phenyl)-thiophen-2-yl]-acetic acid methyl ester (87)
[0216] Reaction scheme 11
[0217]
[0218] Step 1: (The following reactions were carried out under anhydrous nitrogen atmosphere) Thiophen-2-yl-acetic acid methyl ester (85) (2.0 g, 12.8 mmol) was dissolved in anhydrous chloroform (9.0 mL) and glacial acetic acid (9.0 mL) , N-bromosuccinimide (2.3 g, 13.0 mmol) was added in portions and the mixture was stirred at room temperature for 3 days, water was added to the reaction mixture, the layers were separated, the aqueous layer was extracted with dichloromethane, and the combined organic The layer was washed several times with 1M NaOH aqueous solution and water, then once with brine and washed with Na 2 SO 4 Drying and purification of the crude product by preparative radial chromatography (CyH / EtOAc 5+1] afforded (5-bromo-thiophen-2-yl)-acetic acid methyl ester (86) as a yellow oil (2.46 g, 81 %), which was used without further purifi...
Embodiment B
[0221] Preparation of 5-(2-amino-phenyl)-thiophene-2-carboxylic acid methyl ester (90)
[0222] Scheme 12
[0223]
[0224] Step 1: LE23 5-Bromo-thiophene-2-carboxylic acid (88) (1.50 g, 7.24 mmol) was dissolved in methanol (10 mL) and concentrated sulfuric acid (0.39 mL, 7.24 mmol) was added. The reaction mixture was stirred at 75 °C for 20 hours, the mixture was cooled to room temperature, the solvent was removed under reduced pressure, and the residue was dissolved in EtOAc, the organic layer was washed with 5% Na 2 CO 3 The aqueous solution was washed 3 times and the combined aqueous layers were extracted with EtOAc. The combined organic layers were washed with brine and washed with Na 2 SO 4 After drying, the solvent was removed under reduced pressure and the residue was dried under oil pump vacuum without further purification to afford the ester (89) as a white solid (1.48 g, 92%). 1 H NMR (400MHz, CDCl 3 ): 3.88(s, 3H); 4.00(br.s, 2H); 6.73-6.82(m, 2H); 7.13-7....
Embodiment C
[0227] Preparation of 4', 5'-dimethoxy-2'-nitrobiphenyl-3-formyl chloride (95)
[0228] Scheme 13
[0229]
[0230] Step 1: DK006 (the following reactions were carried out under nitrogen atmosphere) Tetrakis(triphenylphosphine)-palladium(0) (200mg, 0.17mmol) and methyl 3-bromobenzoate (91) (1.25mg, 5.81mmol ) was dissolved in DME (12 mL), the reaction mixture was carefully degassed (5 times) and washed with N 2 Purge, add 3,4-dimethoxyphenylboronic acid (1.25 g, 6.85 mmol) and 1M NaHCO 3 aqueous solution (17.7 mL, 17.7 mmol), again the reaction mixture was carefully degassed (5 times) and washed with N 2 After purging, the mixture was stirred at 95 °C for 2 hours, the reaction solution was partitioned between EtOAc and water, and the separated aqueous layer was extracted with EtOAc (4 times). The combined organic layers were washed with brine and washed with Na 2 SO 4 After drying, the crude product was purified by flash chromatography (silica gel, CyH / EtOAc 5+1] to af...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com