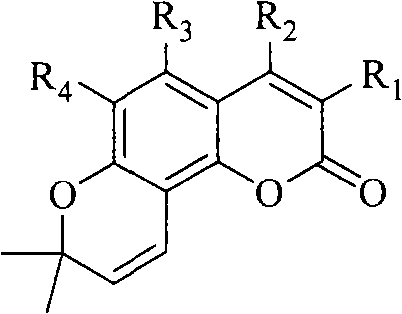
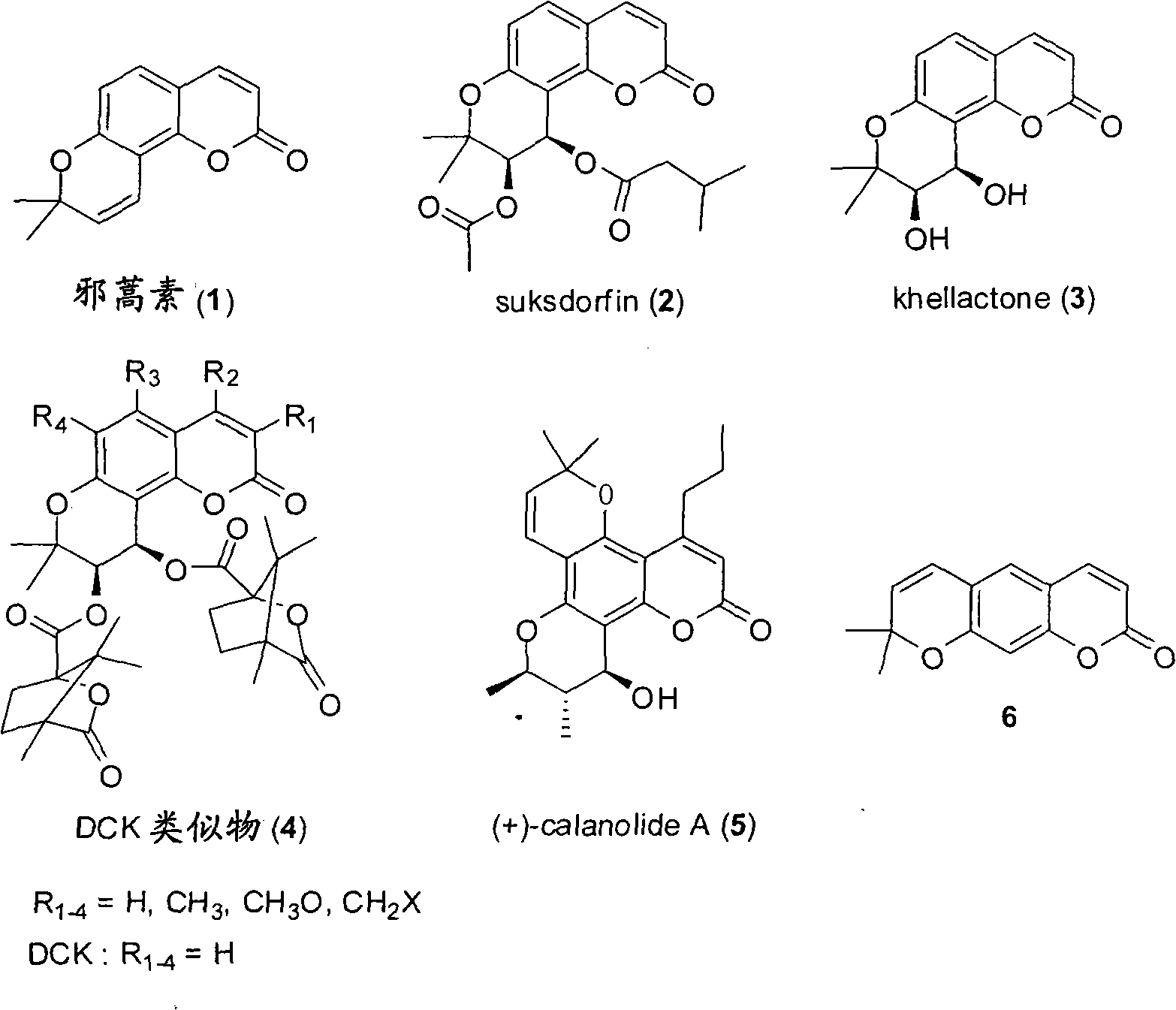
Preparation of amyrolin and derivatives thereof
A technology for the synthesis of eralemisinin and its derivatives, which can solve the problems of harsh high-temperature cyclization conditions, many by-products, and difficult operations, and achieve short reaction time and high overall yield , The effect of simplifying the purification method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 13
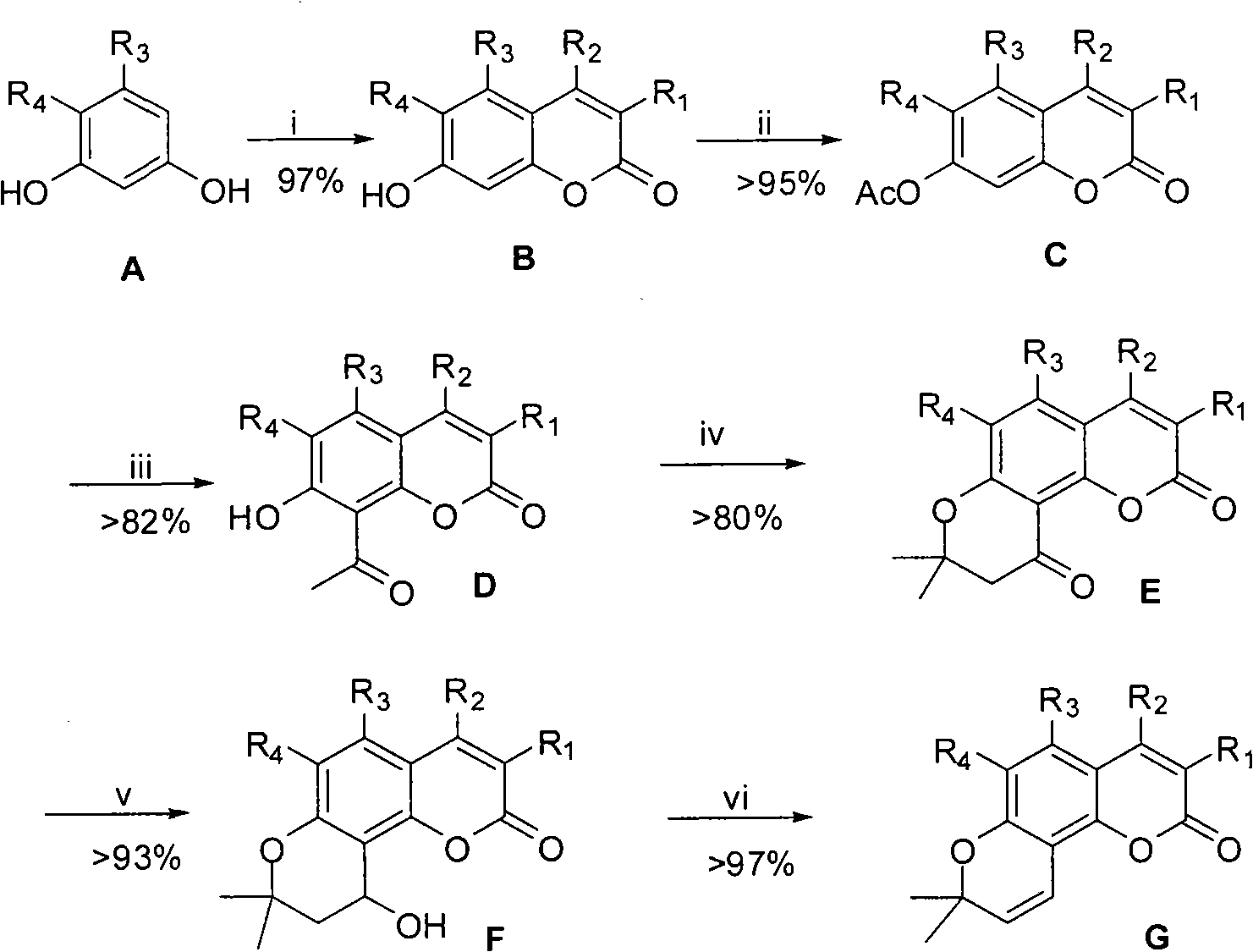
[0037] Embodiment 1.3, the preparation of 4-dimethyl-7-acetoxy coumarin (C1)
[0038] 9.5g (50mmol) of 3,4-dimethyl-7-hydroxycoumarin (B1), 20mL of acetic anhydride, heated to reflux for 90 minutes, cooled to about 50°C, poured into 150g of ice water under vigorous stirring, and continued to vigorously After stirring, a pale yellow solid precipitated, filtered with suction, and washed with water until neutral. The obtained solid was recrystallized with about 500 mL of ethanol to obtain 11.1 g of pale yellow needle crystals, with a yield of 95.7%. mp 164-165°C; 1 H NMR (CDCl 3 , 400MHz, δppm): 2.21 (3H, s, 3-CH 3 ), 2.34 (3H, s, 4-CH 3 ), 2.39 (3H, s, 7-CH 3 COO), 7.05 (1H, dd, J=2.4 & 8.4Hz, 6-H), 7.08 (1H, d, J=2.4Hz, 8-H), 7.60 (1H, d, J=8.4Hz, 5- h).
Embodiment 2
[0039] The preparation of embodiment 2.4-methyl-7-acetoxy coumarin (C2)
[0040] 8.8g (50mmol) of 4-methyl-7-hydroxycoumarin (B2), 20mL of acetic anhydride, heated to reflux for 90 minutes, cooled to about 50°C, poured into 150g of ice water under vigorous stirring, continued vigorous stirring, and precipitated Pale yellow solid, suction filtered, washed with water until neutral. The obtained solid was recrystallized with about 500 mL of ethanol to obtain 10.5 g of pale yellow needle crystals, with a yield of 96.3%. mp150-151°C (documentation: 150-151°C); 1 H NMR (CDCl 3 , δppm): 2.35 (3H, s, 4-CH 3 ), 2.44 (3H, s, 7-CH 3 COO), 6.28 (1H, s, 3-H), 7.09 (1H, dd, J=2.0 & 8.4Hz, 6-H), 7.12 (1H, d, J=2.0Hz, 8-H), 7.62 ( 1H, d, J=8.4Hz, 5-H).
Embodiment 3
[0041] Example 3.3, Preparation of 4-dimethyl-7-hydroxyl-8-acetylcoumarin (D1).
[0042] 11.02g (47.5mmol) of 3,4-dimethyl-7-acetoxycoumarin (C1) and 25g of anhydrous aluminum trichloride were placed in a mortar, fully ground and mixed, and the mixture was filled into 250mL in a round bottom flask. The oil bath was preheated to 130°C, and put into the flask. Slowly raise the temperature of the oil bath to 170-180°C over two hours and heat at this temperature for 2 hours. Cool to room temperature, add 60g of crushed ice, slowly add 150mL of 5% hydrochloric acid under vigorous stirring, and slowly heat to 110°C to obtain a suspension, keep it warm for 1 hour to complete the decomposition. Cool to room temperature, filter with suction, wash with water until neutral. The obtained solid was recrystallized with 300 mL of ethanol to obtain 9.1 g of yellow needle crystals with a yield of 82.6%. mp 175-176°C; 1 H NMR (CDCl 3 )δppm 2.21 (3H, s, 3-CH 3 ), 2.39 (3H, s, 4-CH 3 ), 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com