2-methoxyestradiol lipidosome freeze-dried injection and preparation method thereof
A technology of methoxyestradiol and methoxyestradiol, which is applied in the field of medicine, can solve the problems of short half-life, low oral bioavailability, poor water solubility, etc., and achieves improved stability, huge economic benefits and social benefits. Effective, high encapsulation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Weigh 20 mg of 2-methoxyestradiol, 500 mg of egg yolk lecithin and 250 mg of cholesterol, dissolve in 20 ml of ether, dissolve 15 mg of poloxamer-188 in 20 ml of PBS buffer solution (phosphate buffer solution), and then Slowly inject the ether phase into the buffer solution at a constant speed at 50°C, and shake off the ether with magnetic stirring to obtain a milky white liposome suspension. Dissolve 1000mg trehalose in the liposome suspension, and then pass it through a high-pressure milk homogenizer three times or Ultrasonic pulverization process obtains liposome, Co-60 sterilizes, sub-packs with vials, freeze-dries to obtain freeze-dried powder injection, freeze-dried powder injection is redissolved with water for injection, and the encapsulation rate is 85%, The average particle size is 200nm, and the Zeta potential is -25mV.
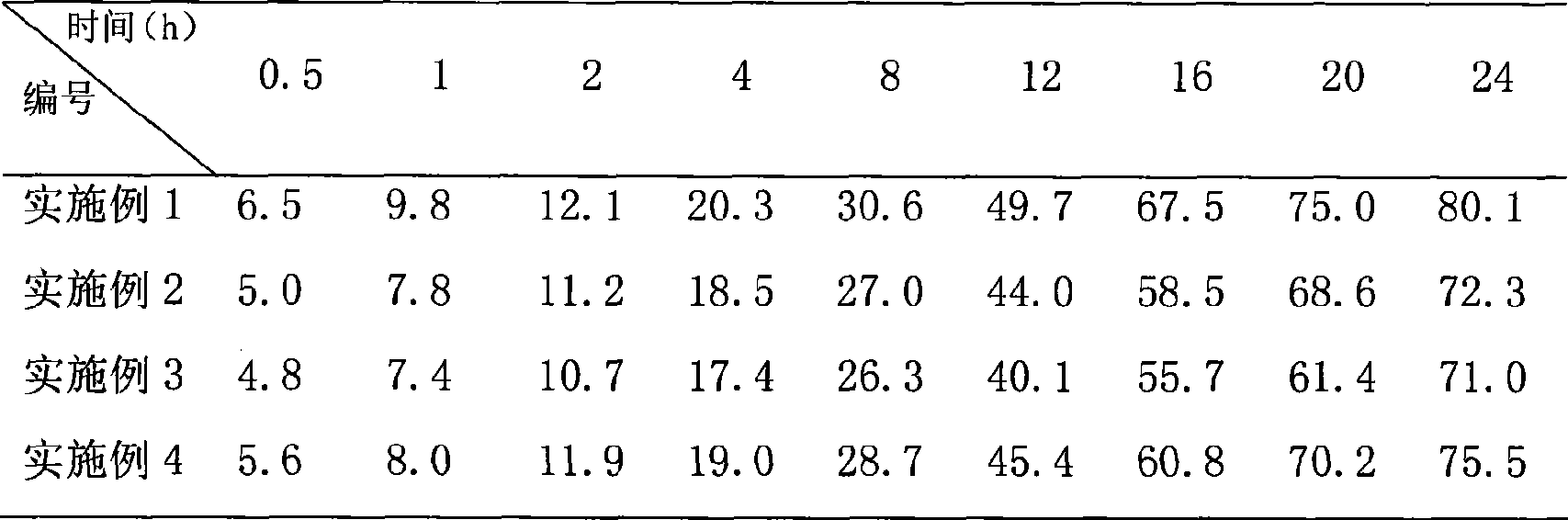
[0021] The in vitro release results of the product of this example are shown in Table 1, and the results show that the preparation has sust...
Embodiment 2
[0023] Weigh 20mg of 2-methoxyestradiol, 500mg of egg yolk lecithin and 150mg of cholesterol, dissolve in 20ml of ether, remove the ether by rotary evaporation at 37-45°C, and add 20ml of citric acid-sodium citrate buffer at pH 6.0 after film formation The solution (containing 15mg poloxamer-188) was hydrated, 500mg sucrose and 500mg lactose were dissolved in the liposome suspension, liposomes were obtained by ultrasonication, sterilized by Co-60 irradiation, and packed in vials , freeze-dried to obtain a freeze-dried powder injection, and the freeze-dried powder injection was reconstituted with water for injection, and the encapsulation rate was measured to be 80%, the average particle size was 340nm, and the Zeta potential was -30mV.
[0024] The in vitro release results of the product of this example are shown in Table 1, and the results show that the preparation has sustained release capability. The distribution results in rats are shown in Table 2, which shows that it has...
Embodiment 3
[0026] Weigh 20 mg of 2-methoxyestradiol, 1000 mg of egg yolk lecithin, and 200 mg of cholesterol, dissolve them in 30 ml of ether, and dissolve in 20 ml of carbonic acid-sodium carbonate buffer solution (containing 25 mg of poloxamer-188) at pH 6.0. Evaporate the organic solvent on a rotary film under reduced pressure to make it into a colloidal state, and continue the rotary evaporation to form a milky white suspension. Dissolve 1000mg of glucose in the liposome suspension, and reduce the particle size through a high-pressure milk homogenizer three times or ultrasonic pulverization to obtain liposomes, which are sterilized by Co-60 irradiation, subpackaged in vials, and freeze-dried The freeze-dried powder injection was obtained, and the freeze-dried powder injection was reconstituted with water for injection. The measured encapsulation rate was 55%, the average particle diameter was 208nm, and the Zeta potential was -35mV.
[0027]The in vitro release results of the product...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com