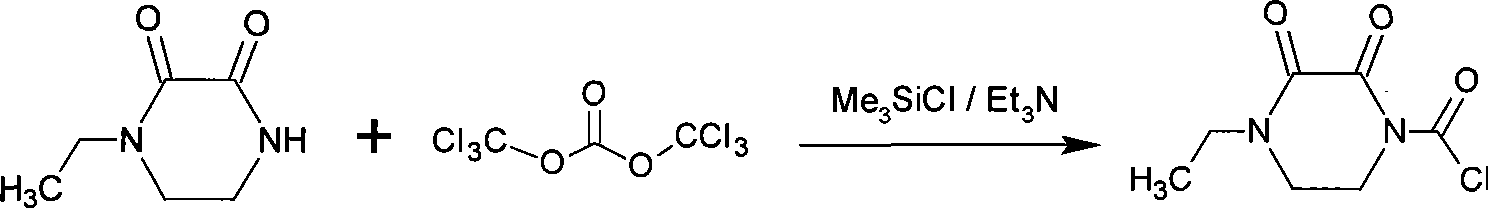Synthesis of 4-ethyl-(2,3-dioxo piperazinyl)formyl chloride-1 and preparation of crystal thereof
A technology of dioxopiperazine and formyl chloride is applied in the field of preparation of 4-ethyl-formyl chloride-1 crystallization, which can solve the problems of inability to be placed, not easy to commercialize, poor solid stability, etc., and achieves stable quality and high quality. The effect of high and short synthesis process route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Add 700L dichloromethane and 80Kg (0.563kmol) 4-ethyl-2,3-dioxypiperazine into a dry 1000L enamel reaction kettle, start stirring, cool to 5°C, and dropwise add 67.3Kg (0.62kmol) Tris Methyl chlorosilane, after stirring for 30 minutes, add 62.7Kg (0.62kmol) triethylamine solution dropwise at the same temperature, keep it warm for 1h after the dropwise addition, continue cooling to -30°C, add 55Kg (0.185kmol) bis- (Trichloromethyl)carbonate, control the temperature rise during the addition process not to exceed 5°C. After the addition, the temperature was naturally raised to room temperature, and the reaction was incubated for 1 h, and the insoluble matter was removed by filtration.
[0022] The filtrate was returned to the reaction kettle, cooled to -10°C, and 400Kg dipropyl ether was added dropwise. After the crystals were precipitated, the addition of dipropyl ether was temporarily stopped, stirred until a large amount of crystals were precipitated, then all the remai...
Embodiment 2
[0025] Add 28.4g (0.2mol) 4-ethyl-2,3-dioxypiperazine and 300mL chloroform in sequence to a dry 500mL three-necked flask, start stirring, cool to 0°C, add 28.2mL (0.22mol) trimethyl Chlorosilane, add 30.5mL (0.22mol) triethylamine solution dropwise through the dropping funnel, keep it warm for 1h after the dropwise addition, cool to -30°C, add 23.7g (0.08mol) bis-(trichloromethyl) Carbonic acid ester, the temperature is controlled not to exceed 5°C during the addition process, and after the addition is completed, the temperature is naturally raised to room temperature, and the reaction is kept for 1.5 hours, and the insoluble matter is removed by filtration.
[0026] Return the filtrate to the three-necked flask, cool down to -10°C, add 200mL of n-hexane, precipitate crystals under stirring, continue to stir for 1h, filter and dry to obtain 30.6g of 4-ethyl-(2,3-dioxopiperazine Base) formyl chloride-1, yield 75%, purity 97.6%.
Embodiment 3
[0028] In a dry 500mL three-necked flask, 300mL of dichloromethane was used instead of chloroform as a solvent, 200mL of petroleum ether (80-120° C.) was used as a crystallization reagent, and other conditions were the same as in Example 2 to obtain 32g of 4-ethyl-(2,3- Dioxopiperazinyl) formyl chloride-1, yield 78.3%, purity 98.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com