Acrylon fluorescent dyeing method
A technology for fluorescent dyeing and fluorescent dyes, which is applied in the dyeing application field of cationic fluorescent dyes on acrylic fibers, can solve the problems such as the application report of textile fabric dyeing, and achieve good linear optical properties and nonlinear optical properties, good water solubility High efficiency and high productivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
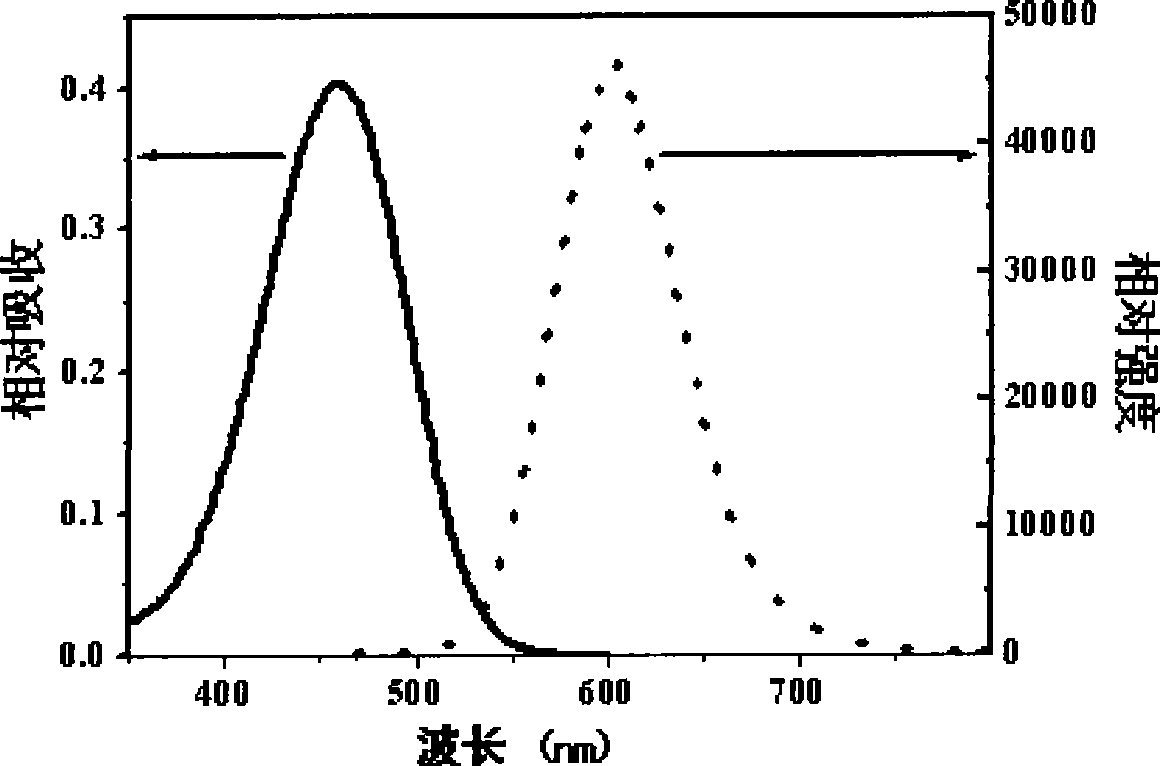
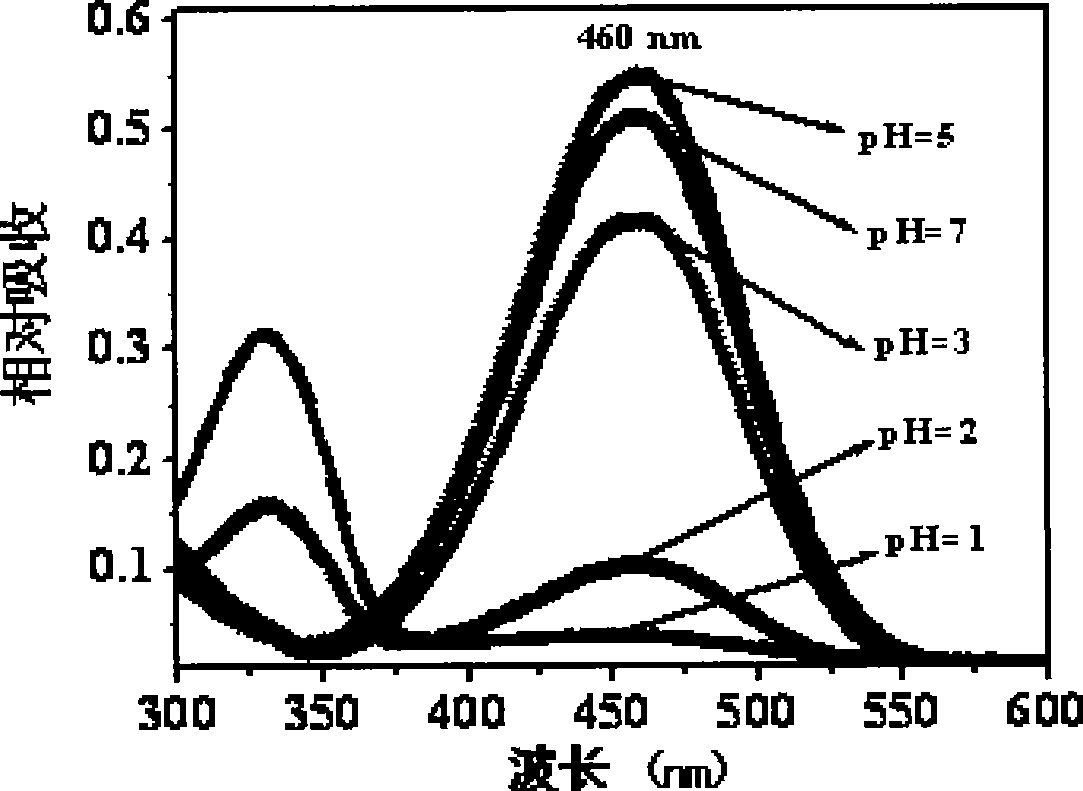
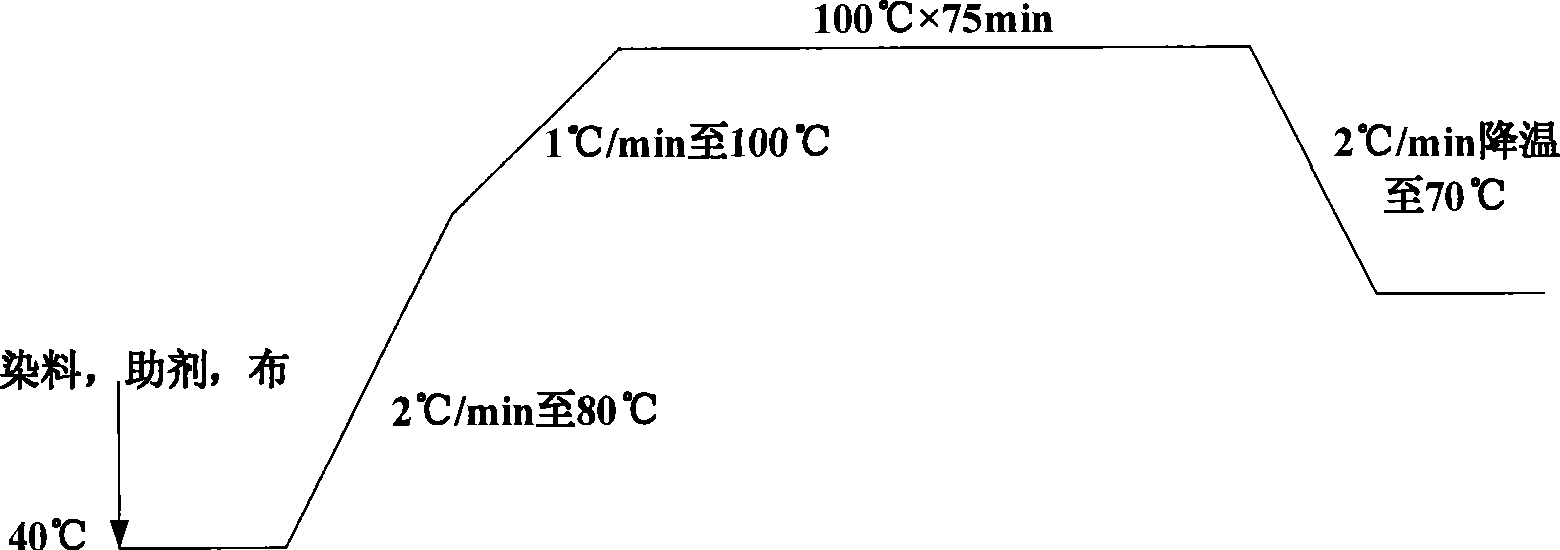
Image
Examples
Embodiment 1
[0030]This embodiment will synthesize trans-4-[4'-(N, N-dihydroxyethylamino) styryl]-N-picoline iodonium salt (abbreviated as DHEASPI-C 1 ), used as a fluorescent dye for acrylic dyeing.
[0031] Step 1, synthesis of 4-N, N-diethanolamine benzaldehyde:
[0032] Weigh N, N-bishydroxyphenethylamine (0.15mol, 27.189g) and pyridine (0.45mol, 36.43mL) and acetic anhydride (0.45mol, 42.48mL) in a round bottom flask, under N 2 Under protection, reflux and stir the reaction for 5 hours. After the reaction, the solution is poured into ice water, purified with ethyl acetate, and anhydrous MgSO 4 After drying, N,N-diacetoxyphenethylamine was obtained. POCL 3 (0.0876mol, 9.06mL) was poured into the constant pressure funnel, and then 2 It was dropped into DMF (0.365mol, 31.63mL) in an ice-bath environment while stirring under protection. After the drop was completed, it was left at room temperature for 30min, and then N,N-diacetoxybenzene dissolved in a small amount of DMF (0.365mol, 3...
Embodiment 2
[0040] Synthesis of trans-4-[4'-(N, N-dihydroxyethylamino) styryl]-N-n-butyl pyridinium bromide (abbreviated as DHEASPBr-C 4 ) as a fluorescent dye for acrylic dyeing.
[0041] Step 1: According to the method provided in step 1 of Example 1, 4-N, N-diethanolamine benzaldehyde was synthesized.
[0042] Step 2: Dissolve 0.01mol (2.3g) of p-methyl-N-n-butylpyridinium bromide in 100mL of absolute ethanol in a flask, and then weigh 0.01mol (2.1g) of the above-mentioned 4-N,N-di Ethanolamine benzaldehyde was added thereto, a few drops of piperidine was added dropwise as a catalyst, and the reaction was stirred for one day at reflux temperature. After removing the solvent, the product was purified by silica gel column chromatography to obtain product-4-[4'-(N, N-dihydroxyethylamino) styryl]-N-n-butylpyridinium bromide (DHEASBr- C 4 ), the yield was 76%, and the decomposition temperature was 308.4°C. NMR 1 H NMR (DMSO-d 6 , 400MHz) δ: 8.72 (2H, Py-CH, d, J6.0Hz), 8.02 (2H, Py-CH...
Embodiment 3
[0050] Synthesis of trans-4-[4'-(N, N-dihydroxyethylamino) styryl]-N-n-octylpyridinium bromide (abbreviated as DHEASPBr-C 8 ) as a fluorescent dye for acrylic dyeing.
[0051] Step 1: According to the method provided in step 1 of Example 1, 4-N, N-diethanolamine benzaldehyde was synthesized.
[0052] Step 2: Dissolve 0.01mol (2.9g) of p-methyl-N-n-octylpyridinium bromide in 100mL of absolute ethanol in a flask, then weigh 0.01mol (2.1g) of the above-mentioned 4-N,N-di Ethanolamine benzaldehyde was added thereto, a few drops of piperidine was added dropwise as a catalyst, and the reaction was stirred for one day at reflux temperature. After removing the solvent, the product was purified by silica gel column chromatography to obtain the product-4-[4'-(N, N-dihydroxyethylamino) styryl]-N-n-octylpyridinium bromide (DHEASPBr- C 8 ). The yield is 69%, and the decomposition temperature is 298.0°C. NMR 1 H NMR (DMSO-d 6 , 400MHz) δ: 8.80 (2H, Py-CH, d, J6.9Hz), 8.08 (2H, Py-CH,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com