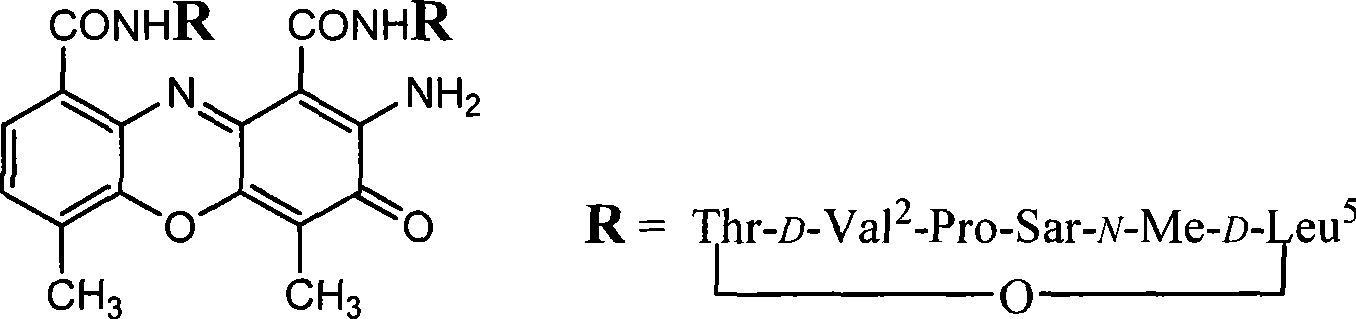New high-efficiency low-toxicity actinomycin type D antineoplastic compound
A high-efficiency and low-toxicity technology of actinomycin, applied in the field of new analogs of actinomycin D, can solve the problems of high toxicity and decreased anti-tumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1: [D-MeLeu 5 ] 2 Synthesis of AMD
[0019] 1 Dissolve 0.63 g (2.26 mmol) of the compound N-benzyloxycarbonyl-N-methylglycine tert-butyl ester in 6 ml of anhydrous methanol (MeOH), add 80 mg of 10% Pd / C, stir for 2 hours after hydrogenation, and filter. Concentrate, add Z-Pro 0.535g (2.15mmol) to the residue, dissolve with dry dichloromethane (DCM) and cool to -10°C, add 0.44g (2.15mmol) of DCC in dichloromethane solution, at -10 After reacting at ℃ for 2 hours, react overnight at room temperature. The DCU generated by the reaction was filtered off, the filtrate was concentrated, and the residue was dissolved in ethyl acetate (EtOAc), followed by 10% citric acid aqueous solution, 10% sodium bicarbonate (NaHCO 3 ) aqueous solution, saturated sodium chloride (NaCl) aqueous solution, and anhydrous sodium sulfate (NaCl) 2 SO 4 )dry. After filtration, the solvent was evaporated under reduced pressure, and the crude product was subjected to silica gel column ...
Embodiment 2
[0027] Embodiment 2: [D-MeLeu 5 ] 2 In vitro antitumor activity experiment of AMD
[0028] The compounds of the present invention and actinomycin D parent, through the human liver cancer cell BEL-7402, human breast cancer cell MCF-7, tongue cancer cell TB, human liver cancer cell HepG-2, human gastric adenocarcinoma cell SGC-7901 and other tumors The cell lines were tested and compared with anti-tumor activity in vitro by MTT method. The MTT method is the colorimetric method of tetramethylazolium salt microenzyme reaction. MTT is a thiazole salt, the chemical name is 3-(4,5-dimethyl-2-thiazole)-2,5-diphenyltetrazolium bromide, and its aqueous solution is yellow-orange. After the oxidized MTT enters the cell, it is reduced by dehydrogenase to form blue-purple formazan (Formazan) particles, which are deposited in or around the cell. Since the production of formazan is related to the number of cells, the type of cells, and the time of action. When the type of cells and the a...
Embodiment 3
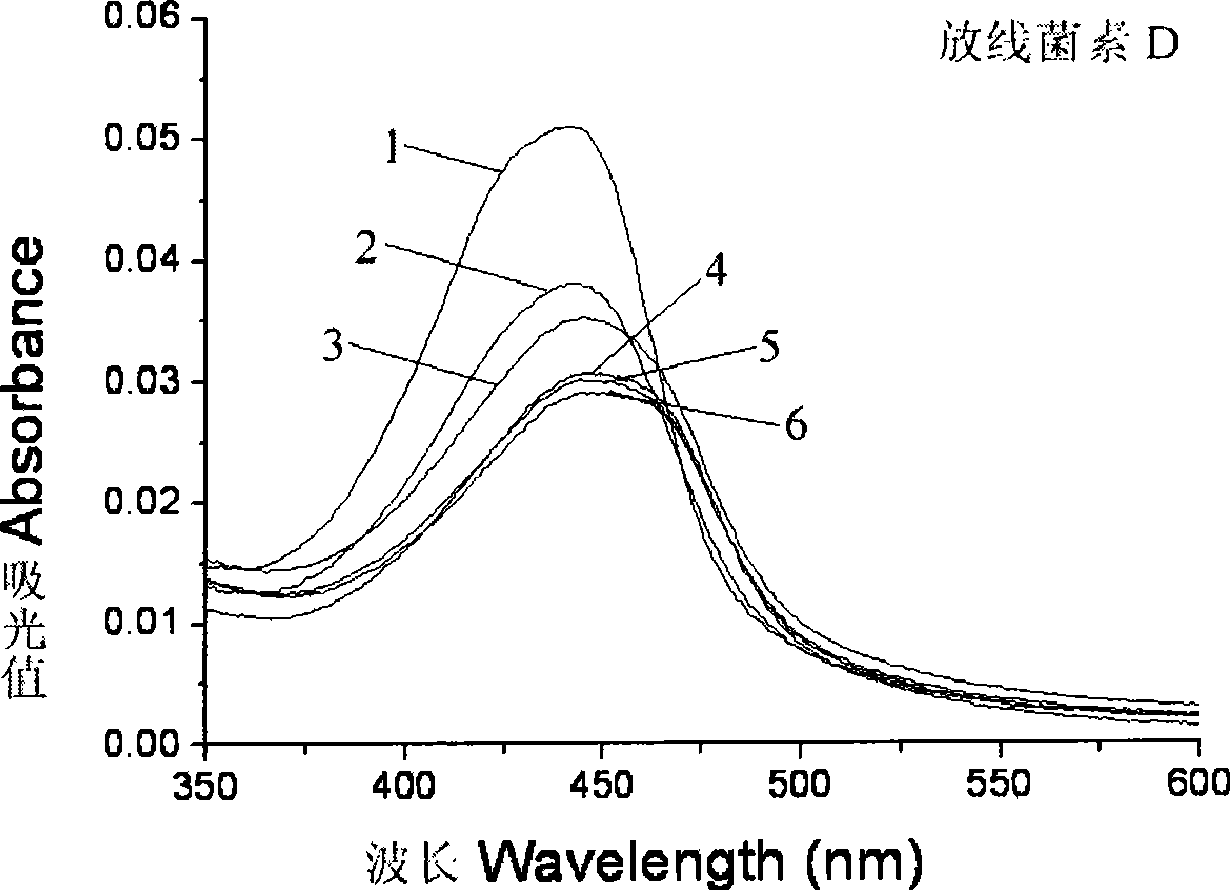
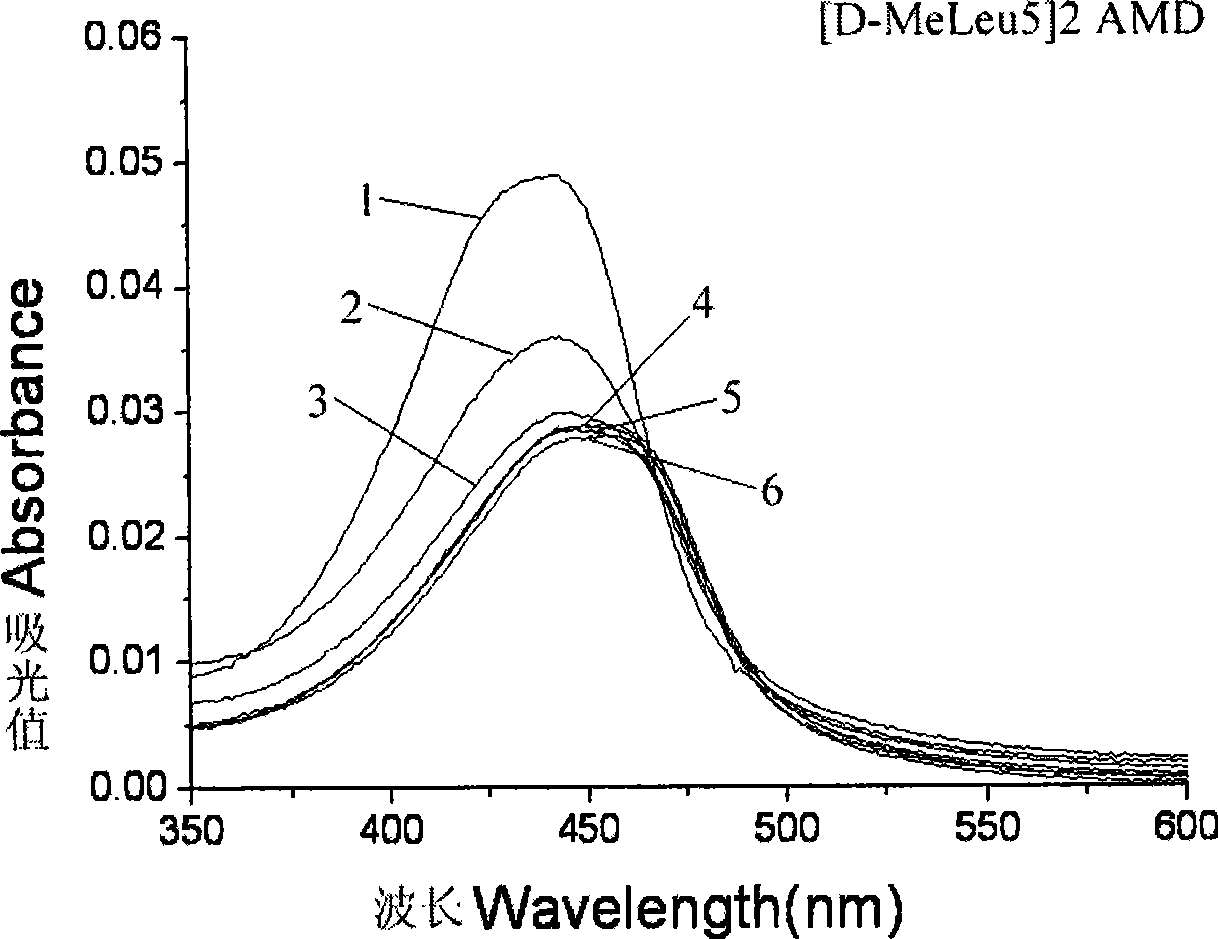
[0040] Embodiment 3: [D-MeLeu 5 ] 2 Study on the DNA binding activity of AMD
[0041] When actinomycin D intercalates with DNA, the visible spectrum shows red shift and color reduction effect; when actinomycin D analogues bind to DNA in a similar way to the parent, and the intercalation is the main factor, the ultraviolet-visible The spectrum showed red shift and color reduction effect; when the binding method of actinomycin D analogs and DNA was dominated by hydrophobic stacking, the ultraviolet-visible spectrum showed only color reduction effect, or had a weak red shift. By research [D-MeLeu 5 ] 2 UV-Vis spectra of AMD and actinomycin D precursors bound to DNA can be studied [D-MeLeu 5 ] 2 Changes in the way AMD binds to DNA.
[0042] I Materials and methods: Instrument: HP-8453 UV-Vis spectrophotometer of Hewlett-Packard Company. Materials: Calf thymus DNA was purchased from Sigma, A260 / A280>1.8 was detected before use, and the purity met the experimental requirement...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com