Method for preparing andrographolide solid dispersion
A technology of andrographolide and solid dispersion, which is applied in the direction of medical preparations containing active ingredients, pharmaceutical formulas, organic active ingredients, etc., and can solve the problem of poor thermal stability of polyethylene glycol-based solid dispersions and difficulty in regrinding , drug dissolution rate decline and other issues, to achieve the effect of being suitable for large-scale production, reducing the loss of pharmacological activity, and low temperature in the preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] A preparation method of a solid dispersion of andrographolide, comprising the following steps:
[0023] (1) 5g andrographolide, 35g polyvinylpyrrolidone k30, 7.5g Tween 80 are dissolved in 250ml absolute ethanol;
[0024] (2) The mixture obtained in step (1) was heated in a water bath at 45° C. to vacuumize to obtain a viscous substance, which was dried to a constant weight and pulverized to obtain a solid dispersion powder of andrographolide.
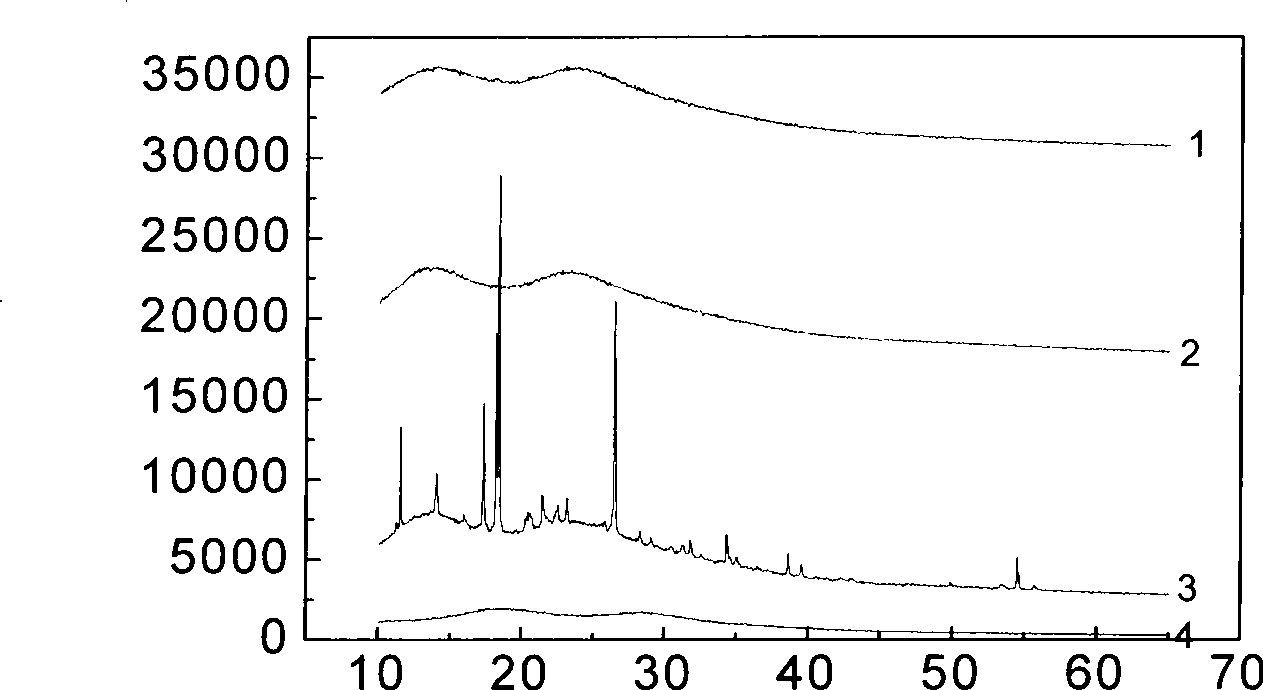
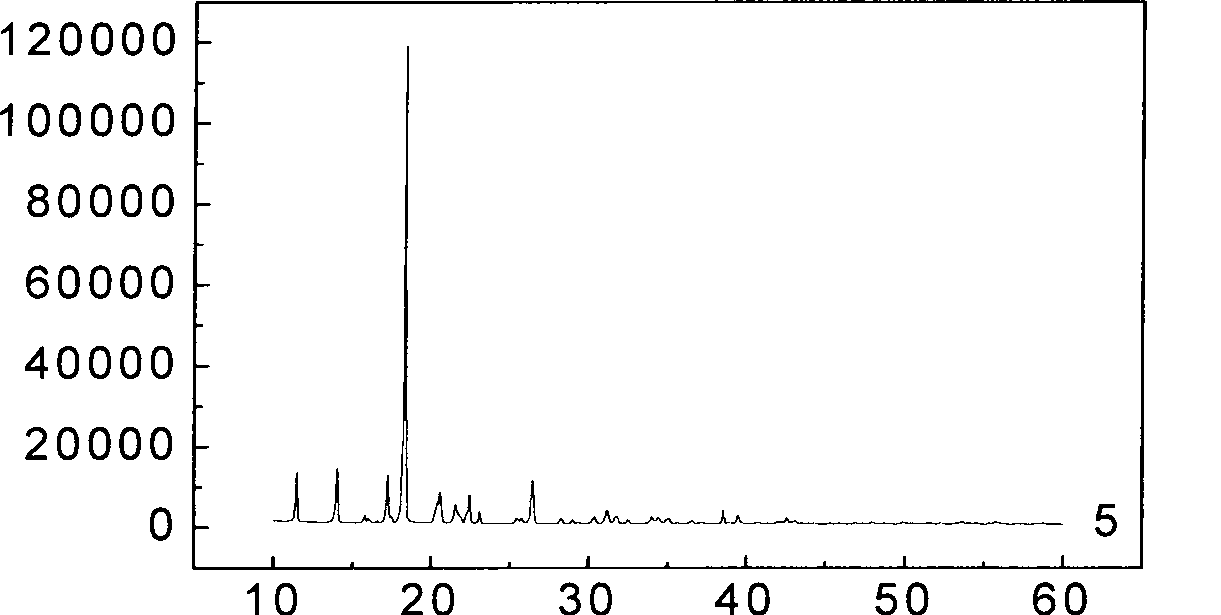
[0025] Andrographolide (Andro) bulk drug (over 80 mesh), PVPk30, Tween 80, the physical mixture of bulk drug and carrier and the X-ray diffraction curve of Andro solid dispersion are as shown in Figure 1. It can be seen from curve 5 in Figure 1 that the Andro API has a very strong crystal diffraction peak at 17°-18°. However, the diffraction pattern (curve 2) of the carrier PVPk30 has only two very weak diffuse peaks, indicating that the carrier PVPk30 exists in a microcrystalline or amorphous state. In addition, because the d...
Embodiment 2
[0028] Three andrographolide solid dispersions with different PVPk30 contents were prepared.
[0029] The first part: take 5gAndro, 7.5g Tween 80 and 25g PVPk30;
[0030] The second part: take 5gAndro, 7.5g Tween 80 and 35g PVPk30;
[0031] The third part: Take 5g Andro, 7.5g Tween 80 and 45g PVPk30, dissolve the above three parts in three parts of 250ml absolute ethanol respectively, heat the obtained three parts in a water bath at 45°C, and vacuumize most of the free Evaporate the water and ethanol to obtain a viscous substance, pour it into a watch glass, put it in a drying oven, and dry it for several days, then crush the dried solid dispersion and grind it to 80 meshes to obtain three Andro solid dispersions with different PVPk30 contents. body powder. Their release curves with Andro bulk drug and PEG carrier Andro preparation (Andro dropping pills) in simulated gastric juice (pH=1 hydrochloric acid: dehydrated alcohol=65:35) are as follows: image 3 . Depend on ima...
Embodiment 3
[0033]5gAndro, 45g PVPk30, 5g Tween 80 were dissolved in 250ml absolute ethanol, and the resulting mixture was spray-dried at 60°C to directly obtain white Andro solid dispersion powder, which was dissolved in simulated gastric juice (pH=1 hydrochloric acid: absolute ethanol =65:35) the cumulative dissolution rate reached 100% in 5 minutes.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com