Side chain type sulphonation polyarylether ketone based on naphthalene ring and preparation thereof
A technology for sulfonating polyaryletherketone and polyaryletherketone, which is applied in the field of polymer chemistry and can solve the problems of inability to meet battery usage requirements, water absorption, swelling rate and other performance degradation problems.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
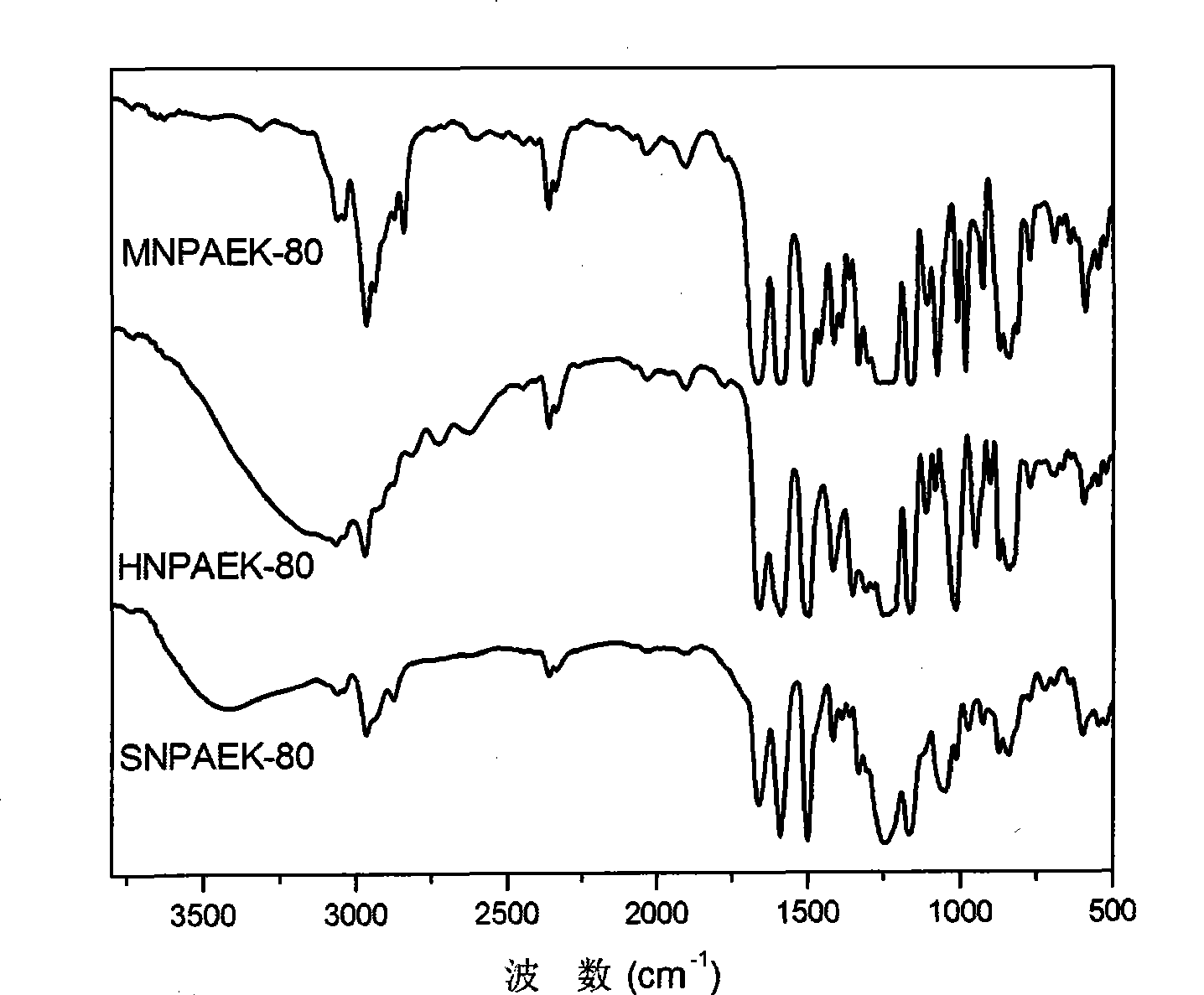
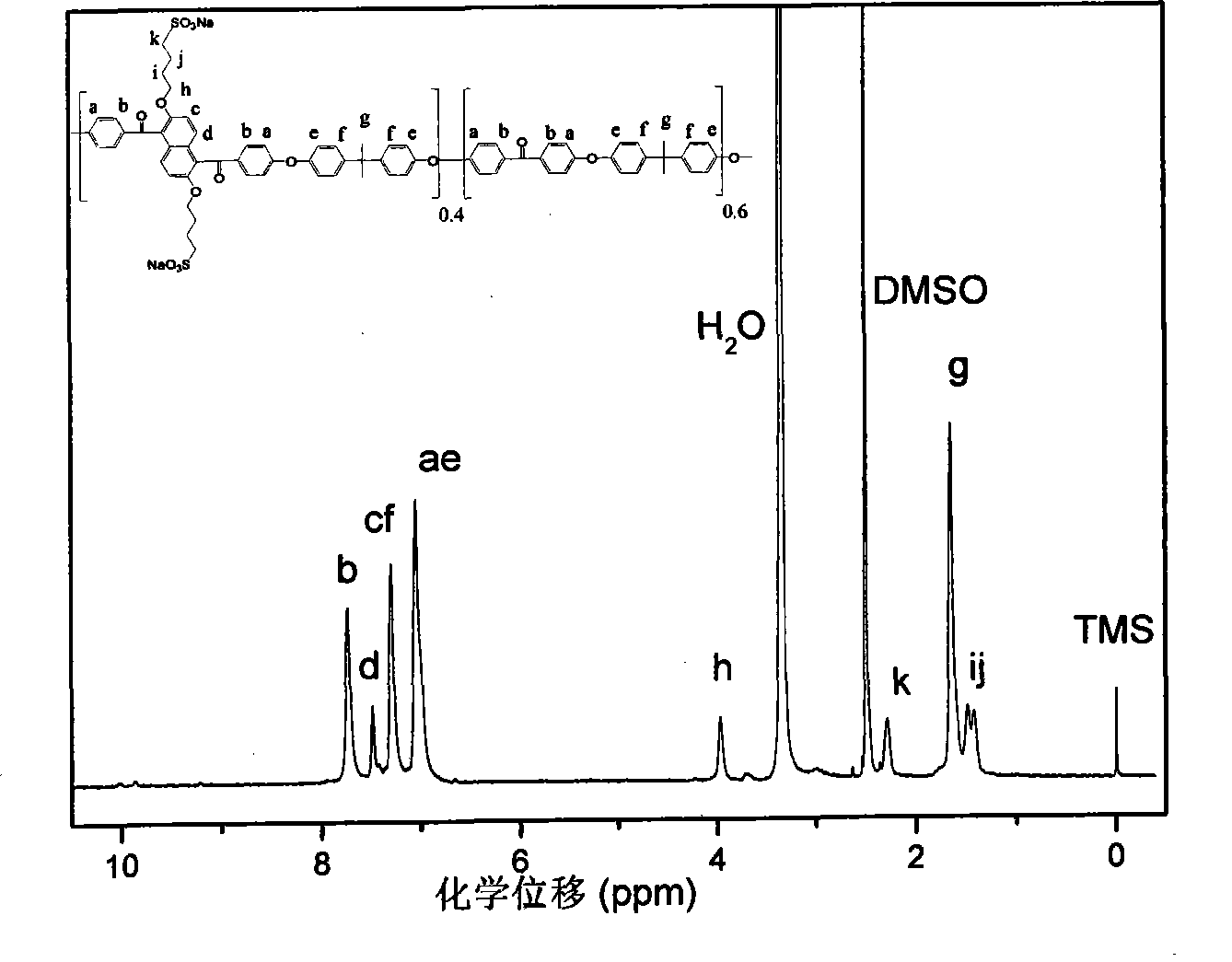
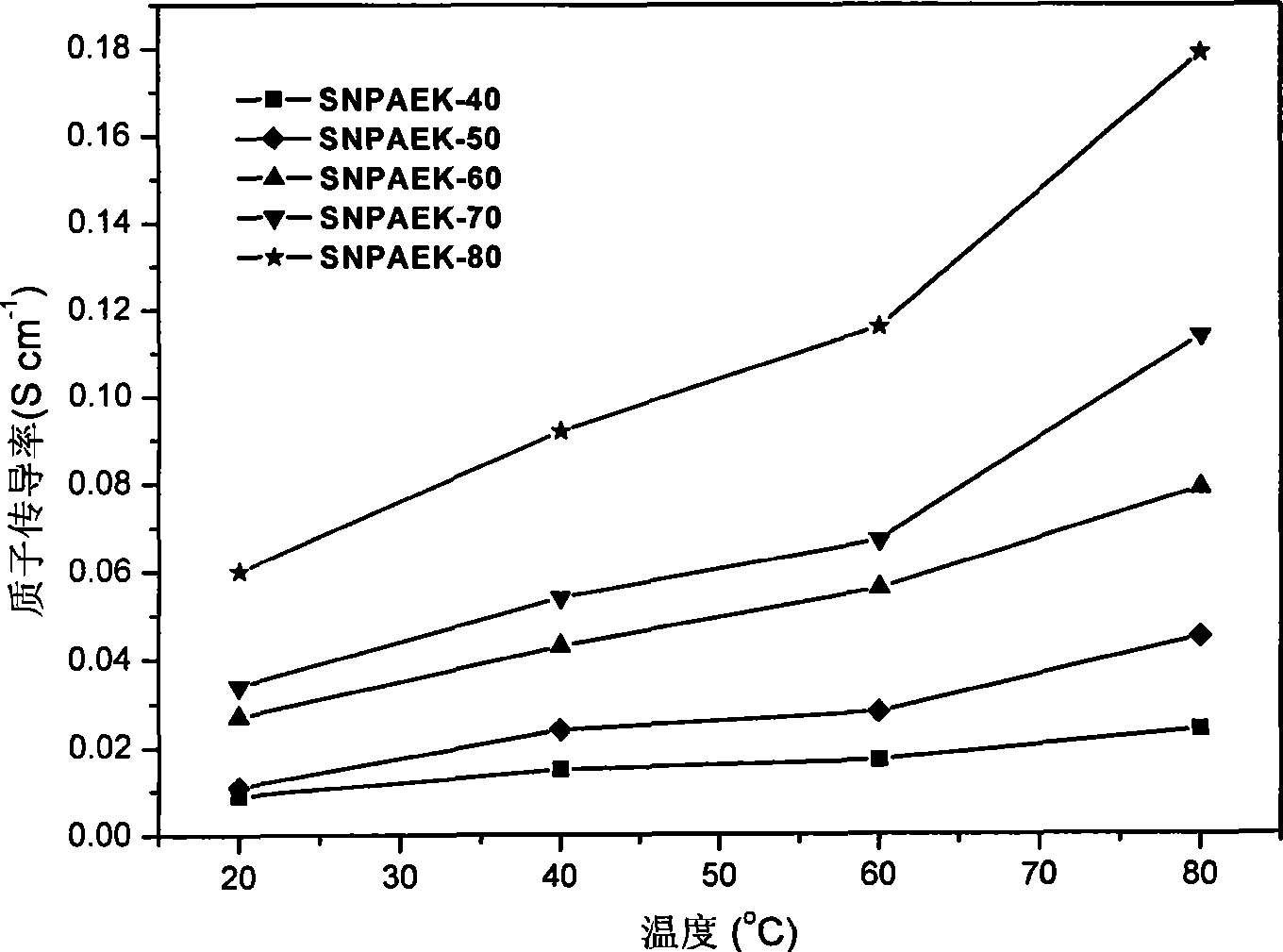
Image
Examples
Embodiment 1
[0041] Example 1: Synthesis of 1,5-bis(4-fluorobenzoyl)-2,6-dimethoxynaphthalene
[0042]In a 500ml three-necked flask, add 0.1mol of 2,6-dimethoxynaphthalene, 300ml of chloroform, and 0.25mol of 4-fluorobenzoyl chloride. After stirring evenly in an ice-water bath under the protection of a nitrogen atmosphere, slowly add 34g of Aluminum trichloride in water, react at 0°C for 16 hours. The system is a purple-black solution. Pour the obtained solution into 1000ml of hydrochloric acid solution with a concentration of 0.5mol / L, soak for 2 hours, wash 5 times with water, collect the oily liquid in the lower layer, and add cyclohexane as a precipitant to the oily liquid to obtain a white Powdery solid; use N,N-dimethylformamide and water at a volume ratio of 10:1 as a mixed solvent for recrystallization to obtain 1,5-bis(4-fluorobenzoyl)-2,6-dimethoxy Basenaphthalene, the yield is 82%, and the melting point is 262°C.
Embodiment 2
[0043] Embodiment 2: Synthesis of dimethoxynaphthalene content is 100% bisphenol A type polyaryletherketone containing dimethoxynaphthyl group
[0044] Add 0.05mol of 1,5-di(4-fluorobenzoyl)-2,6-dimethoxynaphthalene, 0.05mol of bisphenol A and 0.0525mol of anhydrous potassium carbonate into a three-port tube equipped with mechanical stirring and a thermometer in the bottle. Under the protection of nitrogen, use 145ml of N-methylpyrrolidone as a solvent and 30ml of toluene as a water-carrying agent, carry water at 140°C for 4 hours, then distill off the toluene, heat up to 180°C for 8 hours, and then pour the reaction mixture into water. Pulverize and filter with a pulverizer, boil and filter the solid directly with distilled water, repeat 6 times, and dry in an oven to obtain 31g of bisphenol A type polyarylene containing dimethoxynaphthyl groups with a dimethoxy content of 100%. ether ketone. The yield was 94%, and its glass transition temperature was 229°C.
Embodiment 3
[0045] Embodiment 3: Synthesis of dimethoxynaphthalene content is 80% bisphenol A type polyaryletherketone containing dimethoxynaphthyl group
[0046] 0.04mol 1,5-bis(4-fluorobenzoyl)-2,6-dimethoxynaphthalene, 0.01mol 4,4'-difluorobenzophenone, 0.05mol bisphenol A and 0.0525mol Potassium carbonate water was added to a three-necked flask equipped with a mechanical stirrer and a thermometer. Under the protection of nitrogen, use 135ml of N-methylpyrrolidone as a solvent and 28ml of toluene as a water-carrying agent, carry water at 140°C for 4 hours, then distill off the toluene, heat up to 180°C for 8 hours, and then pour the reaction mixture into water. Pulverize and filter with a pulverizer, boil and filter the solid directly with distilled water, repeat 6 times, and dry in an oven to obtain 31 g of bisphenol A-type dimethoxynaphthyl-containing aggregates with a dimethoxynaphthalene content of 80%. Aryl ether ketone. The yield was 93%, and its glass transition temperature wa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com