Surface hydrophilic modification of polystyrene material and product
A polystyrene and hydrophilic modification technology, which is applied in the direction of material separation, analysis materials, measuring devices, etc., can solve the problems of unsuitability and inability to effectively cover the hydrophobic surface of polystyrene, and achieve the improvement of hydrophilicity and non-specificity The effect of reducing the adsorption capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
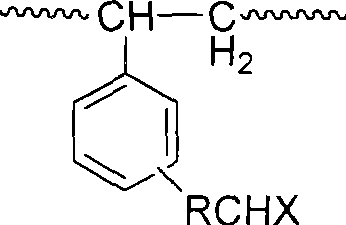
[0041] A Chloroacetylation of polystyrene
[0042] Add 0.8g of ordinary polystyrene plate, 20ml of dichloromethane, 1.2g of anhydrous aluminum trichloride to a 50ml flask, stir with a magnet, the solution turns yellow, then add 0.8ml of chloroacetyl chloride dropwise, and react in an oil bath at 30°C 5h. The reaction solution was suction-filtered in anhydrous state, the separated solid matter was quickly poured into glacial hydrochloric acid and stirred, filtered again and washed with deionized water until neutral, finally washed and filtered with absolute ethanol, and dried in a vacuum oven.
[0043] Chloroacetylated polystyrene after drying is measured chlorine content with sodium hydroxide melting method, and infrared spectrum (FTIR) analysis shows, sample compares in 1683cm before reaction. -1 A strong carbonyl stretching vibration absorption peak connected to the benzene ring appears at 645cm -1 A strong chlorine atom (-Cl) stretching vibration peak appears at the cente...
Embodiment 2
[0047] A Chloroacetylation of polystyrene
[0048] Add 0.8g of polystyrene microspheres, 24ml of carbon disulfide, and 1.06g of anhydrous aluminum trichloride to a 50ml flask, stir with a magnet, and the solution turns yellow, then add 0.3ml of chloroacetyl chloride dropwise, and react in an oil bath at 50°C for 3h. The reaction solution was suction-filtered in anhydrous state, the separated solid matter was quickly poured into glacial hydrochloric acid and stirred, filtered again and washed with deionized water until neutral, finally washed and filtered with absolute ethanol, and dried in a vacuum oven. The chlorine content of the modified microspheres was 12.02%.
[0049] B Chloroacetylated polystyrene coupled PVA
[0050] Swell 1.0 g of chloroacetylated polystyrene microspheres obtained above with 5 ml of DMF overnight, add 35 ml of PVA9K-10K DMF solution to ensure that the PVA concentration in the reaction system is 20 mg / ml, and add tetrabutyl iodide Ammonium 0.76g, pot...
Embodiment 3
[0052] A Bromoacetylation reaction of polystyrene
[0053] Add 1g of polystyrene microspheres, 24ml of carbon disulfide, and 1.5g of anhydrous aluminum trichloride into a 50ml flask, stir with a magnet, and the solution turns yellow, then add 0.4ml of bromoacetyl bromide dropwise, and react in an oil bath at 50°C for 3h. The reaction solution was suction-filtered in anhydrous state, the separated solid matter was quickly poured into glacial hydrochloric acid and stirred, filtered again and washed with deionized water until neutral, finally washed and filtered with absolute ethanol, and dried in a vacuum oven. The bromine content of the modified microspheres was 25.54%.
[0054] B bromoacetylated polystyrene coupled PVA
[0055] Swell 0.6g of bromoacetylated polystyrene microspheres obtained above with 5ml DMF overnight, add 35ml of PVA22K DMF solution, ensure that the PVA concentration in the reaction system is 10mg / ml, add cetyltrimethyl Ammonium bromide 0.44g, potassium hy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com