Synthesis method of 4,6-dichloro-5-fluoropyrimidine compound
A production method and a fluoropyrimidine technology are applied in the field of compounds with a pyrimidine structure, can solve the problems of large usage amount, many three wastes, difficult operation and the like, and achieve the effects of short reaction time, high yield and easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
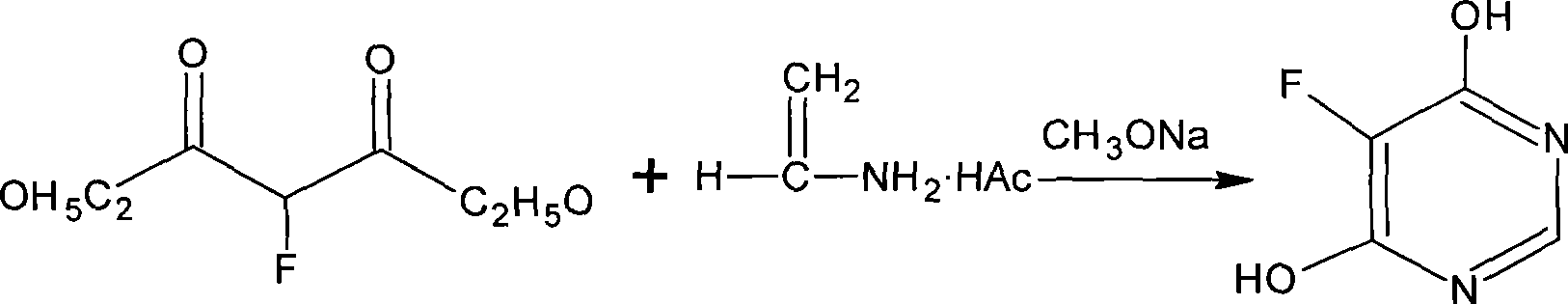
[0028] (1)
[0029]
[0030] Add 108 grams (2.0 mol) of sodium methylate and 1200 milliliters of methanol into a 2000 milliliter reaction flask, then add 208 grams (2.0 mol) of formamidine acetate, and heat up to reflux. 178 g (1.0 mol) of diethyl fluoroacetate was added dropwise, and the addition was completed in about 1 hour. After the addition, the reaction was kept under reflux for 8 hours. After the reflux reaction was completed, the methanol was distilled off under reduced pressure, the residue was added with 1000 ml of water, the pH was adjusted to 3-4 with concentrated hydrochloric acid, and a white solid was precipitated, dried in vacuo at 80°C to obtain 116.0 g of a white solid, the yield was 89.2%, and the purity was determined by HPLC. 96.8%.
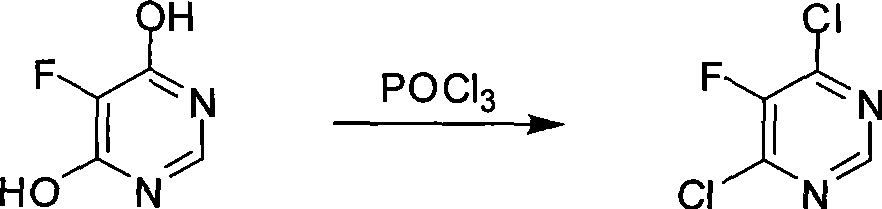
[0031] (2)
[0032]
[0033] Add 195 grams (1.5 mol) of 4,6-dihydroxy-5-fluoropyrimidine to a 2000 ml reaction flask, 1200 ml toluene, 275 ml (3.0 mol) of phosphorus oxychloride, slowly add N, N-di 38.0 ml (0.3 mol)...
Embodiment 2
[0035] Except replacing the toluene among the embodiment 1 with trichlorethylene, all the other raw materials, formula, technique and operating procedure are all the same as the embodiment 1. 175.6 g of 4,6-dichloro-5-fluoropyrimidine was obtained. According to the amount of feed and the amount of 4,6-dichloro-5-fluoropyrimidine obtained, the calculated yield was 80.1%, and the purity was above 98%.
Embodiment 3
[0037] The molar ratio of diethyl 2-fluoropropionate, formamidine acetate and sodium methylate in step (1) is 1:1~50:1~50 (example 1:1:1, 1:25:25, 1 :50:50).
[0038] Tertiary amine catalysts are trimethylamine, triethylamine, 1,8-diazabicyclo[5.4.0]undec-7-decene, 1,4-diazabicyclo[2.2.2]octane, tetramethyl One or more of ethylenediamines, or the salts of the above-mentioned tertiary amines with hydrochloric acid, sulfuric acid, phosphoric acid, formic acid, acetic acid, methanesulfonic acid, benzenesulfonic acid, maleic acid, oxalic acid or succinic acid. The molar ratio of tertiary amine catalyst to 4,6-dihydroxy-5-fluoropyrimidine is 0.01-10:1 (eg 0.01:1, 1:1, 10:1).
[0039] The solvent is benzene, dichloromethane, chloroform or carbon tetrachloride.
[0040] The chlorinating agent is thionyl chloride, sulfuryl chloride, phosphorus trichloride or phosphorus pentachloride.
[0041] All the other are the same as Example 1, the yield is greater than 80%, and the purity is ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com