Novel LXR agonist as well as preparation method and application thereof
A kind of use, the technology of medicinal salt, applied in the field of new compounds, can solve the problem of not being able to regulate the expression of human CYP7A1 gene
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] The cultivation of embodiment 1 bacterial classification
[0064] Slant medium: PDA medium.
[0065] Seed medium: starch 2%, glucose 1%, hot-pressed soybean cake powder 0.2%, malt powder 0.6%, yeast powder 0.3%, NaCl 0.2%, MgSO 4· 7H 2 O 0.1%, CaCO 3 0.2% pH 7.0.
[0066] Fermentation medium: The rice medium is 2.5g of soybean flour per 100g of rice.
[0067] The slant of the fungus CGMCC No.2037 was inoculated in the seed medium, and after 72hr cultivation at 27°C, it was inserted into a 750ml Erlenmeyer flask with a content of 100g of rice medium, and cultivated in a solid medium for 14 days.
Embodiment 2
[0068] Example 2 Isolation and structure of compounds (I), (II) and (III)
[0069] CGMCC No.2037 solid culture 4kg, soaked in 4000ml ethyl acetate for 2 hours, the ethyl acetate layer was washed with anhydrous Na 2 SO 4 After dehydration, concentration and drying, 23.0 g of brown substance was obtained.
[0070] Take 22.0g sample, dissolve and mix the sample, carry out silica gel medium pressure column (φ3.5×50cm) chromatographic separation, the elution condition is 100% petroleum ether to 100% acetone (different ratio) stage elution, collect and combine activity Components were concentrated and dried to obtain a brown solid.
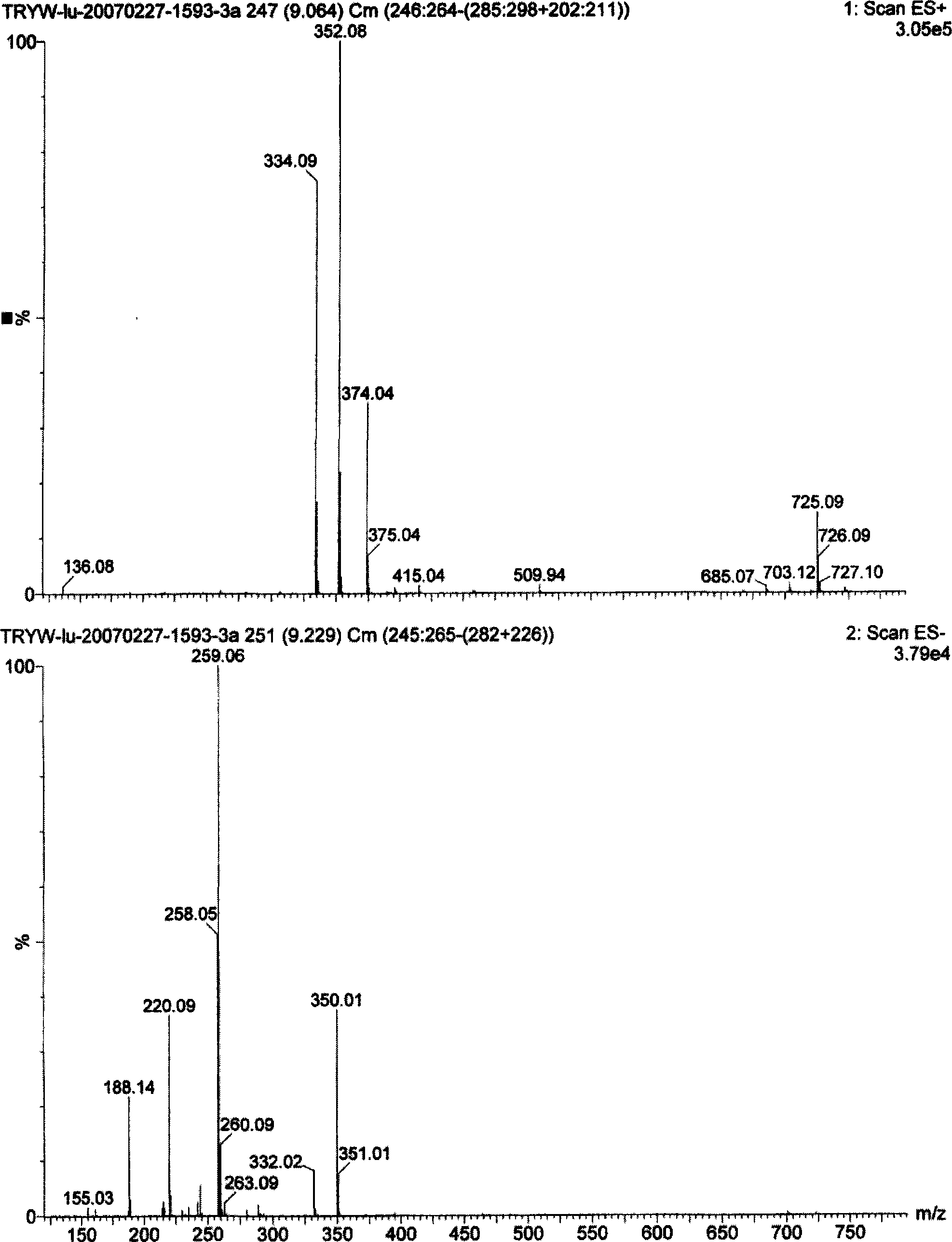
[0071] Take the above-mentioned active substances, use ODS reverse phase column (PHENOMENEX ODS φ 21.2×250mm) to carry out single-component preparation on preparative HPLC [mobile phase is CH 3 CN-(1‰H 3 PO 4 )H 2 O(80:40), the flow rate is 16ml / min, and the detection wavelength is 254nm] to obtain 256.2mg of compound (I), 30.0mg of compound (II) ...
Embodiment 3
[0090] Example 3 Agonistic activity on LXR
[0091] This activity assay is an assay for the transcriptional activation of GAL4-LXR:
[0092] Assay principle: This mechanism utilizes the two main structural domains shared in the structure of LXR: the independence of the ligand-binding domain (LBD) and the DNA-binding domain (DBD) function, and the yeast cell transcription factor GAL4 has a nuclear receptor The ligand-binding domain (LBD) of LXR was fused with the DNA-binding domain (DBD) of yeast cell transcription factor GAL4 to express a chimeric protein, which was co-transfected with a reporter plasmid containing a GAL4-specific response element , to evaluate the activity of LXR ligands by measuring the expression of the reporter gene. According to this principle, the expression plasmid and reporter plasmid were constructed: the LXR-LBD fragment amplified by PCR from liver tissue was connected to the expression vector pBIND by double enzyme digestion to construct the pBIND-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com