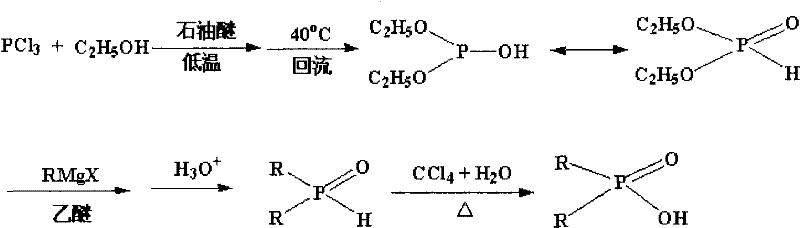
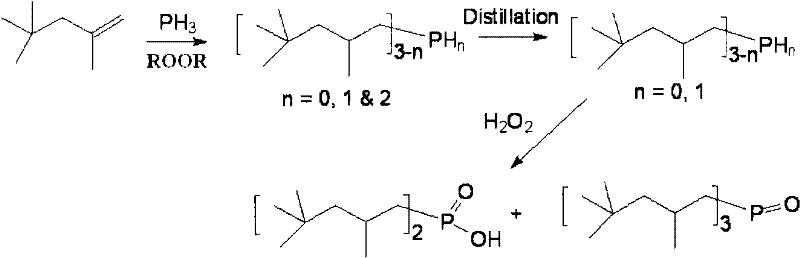
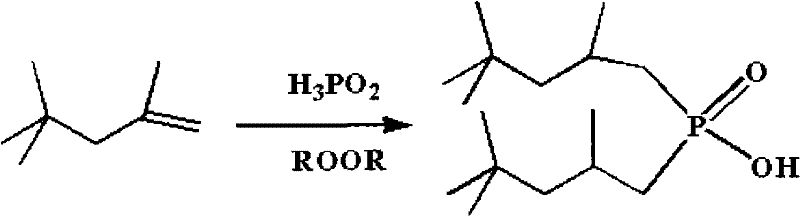
Method for synthesizing dialkyl hypophosphorous acid
The technology of a dialkylphosphinic acid and a synthesis method, which is applied in the field of organophosphorus compound preparation, can solve the problems of difficulty in formation, limitations in compound development and application, complicated reaction conditions and post-processing steps, and reduces the reaction rate and shortens the reaction time. , the effect of improving the purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: the synthesis of dihexylphosphinic acid
[0032] 16.0g of NaH 2 PO 2 ·H 2 O, 16.0g of glacial acetic acid, 32.0g of 1-hexene mixed, put into a stainless steel reactor with Teflon lining, turn on the magnetic stirring, then add 0.73g of benzoyl peroxide and 1.8 g di-tert-butyl peroxide mixture, and finally add 40ml 2,2,4-trimethylpentane to the reactor, seal the reactor, start heating, and react at 120°C for 10 hours while maintaining magnetic stirring. After the reaction, cool down to room temperature naturally, open the reactor, filter the resulting mixture, remove a small amount of solids, transfer the filtrate to a separatory funnel, add 100ml of deionized water, shake, wash, discard the lower aqueous phase, and repeat this process for three times. For four times, the obtained organic phase was rotated at room temperature for one hour to collect 8.2 g of unreacted 1-hexene, and then the temperature was raised to 60°C for rotary evaporation to recover...
Embodiment 2
[0037] Embodiment 2: the synthesis of two (2,4,4-trimethylpentyl) phosphinic acid
[0038] 16.0g of NaH 2 PO 2 ·H 2 0, 16.0g glacial acetic acid, 48.6g diisobutene (containing 80% α-diisobutene and 20% β-diisobutene) mixes, packs in the stainless steel reactor with polytetrafluoroethylene liner, opens magnetic stirring, then goes to A mixture of 0.73 g of benzoyl peroxide and 1.8 g of di-tert-butyl peroxide was added to the reaction kettle, the reaction kettle was sealed, and heating was started, and the reaction was carried out at 140° C. for 15 hours while maintaining magnetic stirring. After the reaction, cool down to room temperature naturally, open the reactor, filter the resulting mixture, remove a small amount of solids, transfer the filtrate to a separatory funnel, add 100ml of deionized water, shake, wash, discard the lower aqueous phase, and repeat this process for three times. For four times, the obtained organic phase was rotated at 60°C for one hour, and 13.0 g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com