Herba Epimedii aglycone liposome and preparation method thereof
A technology of icariin and icariin lipid, which is applied in the directions of liposome delivery, pharmaceutical formulations, medical preparations with inactive ingredients, etc. The problems such as oral absorption and drug effect exertion, to achieve the effect of low cost, simple and easy preparation method, and good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
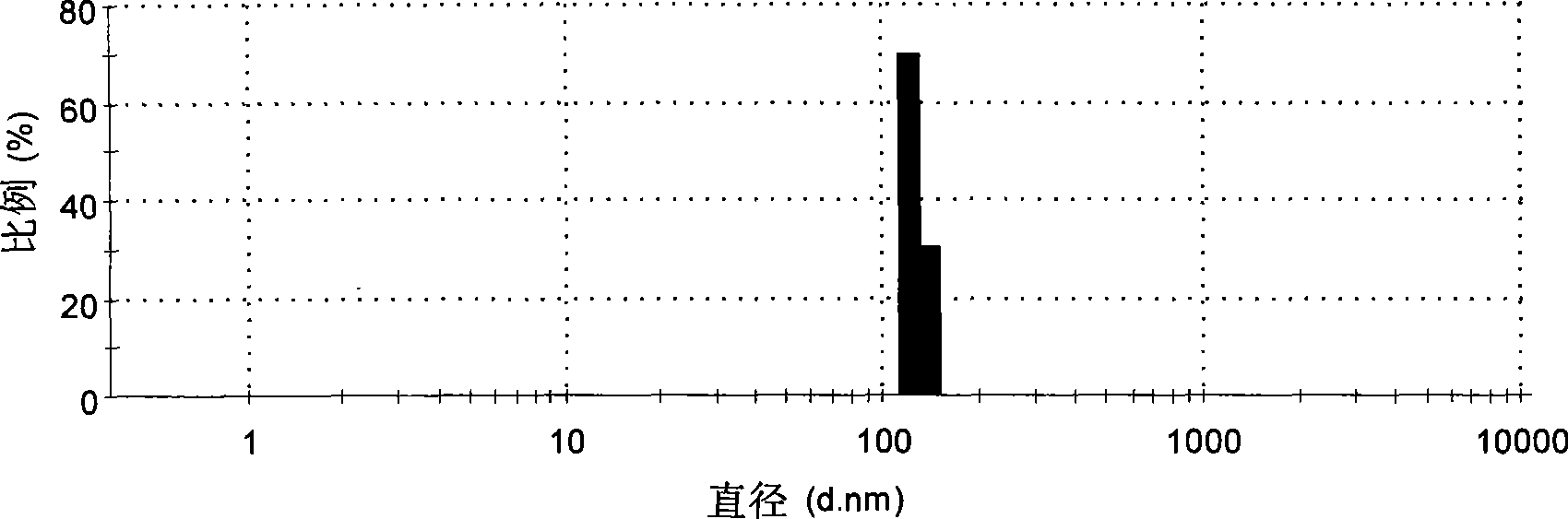
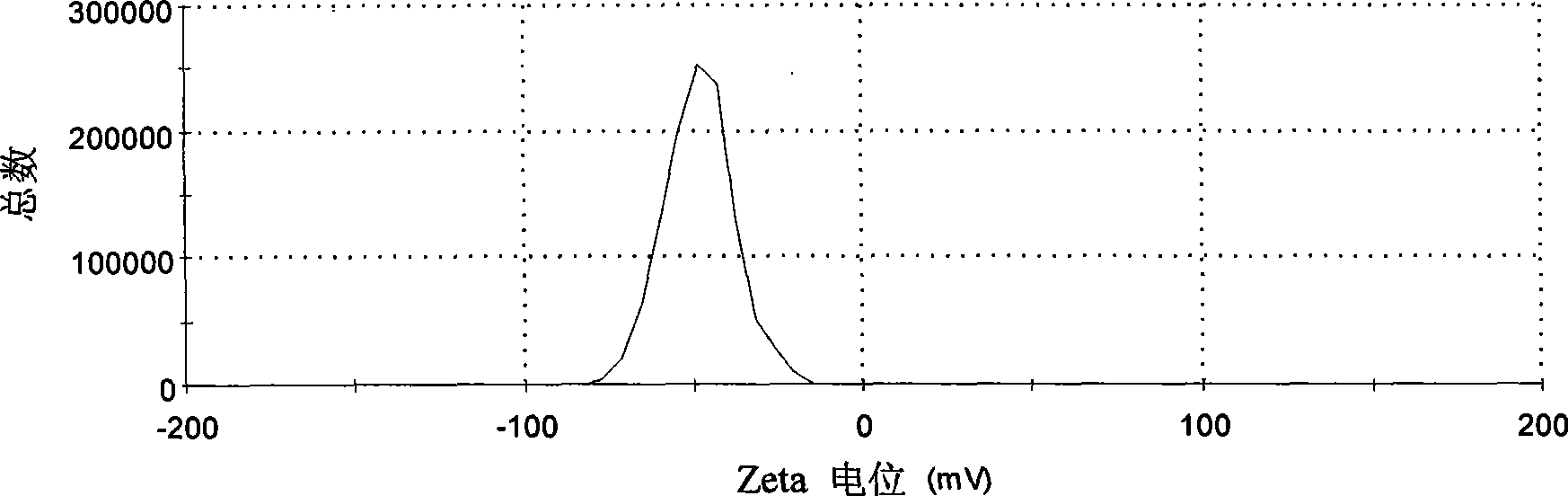
[0042] Weigh 20 mg of icariin, 200 mg of soybean lecithin, and 100 mg of cholesterol, dissolve them in 20 mL of dichloromethane, transfer the dichloromethane solution to a syringe, and slowly inject the dichloromethane solution into 25 mL of 0.5% P-188 (provided by Nanjing Weier Chemical Industry Co., Ltd.) aqueous solution, continue to hydrate for 0.5 hour after the addition, to obtain a liposome suspension. Add 20% mannitol of the volume of the suspension to the liposome suspension, freeze-dry to obtain the icarigenin liposome, see its particle size distribution and Zeta potential figure 1 , figure 2 . The transmission electron microscope images before and after freeze-drying are shown in image 3 , Figure 4 .
Embodiment 2
[0044] Weigh 20 mg of icariin, 200 mg of soybean lecithin, and 100 mg of cholesterol, dissolve them in 10 mL of dichloromethane, transfer the dichloromethane solution to a syringe, and slowly inject the dichloromethane solution into 25 mL of 0.5% P-188 (provided by Nanjing Weier Chemical Industry Co., Ltd.) aqueous solution, continue to hydrate for 0.5 hour after the addition, to obtain a liposome suspension. Add 15% mannitol of the suspension volume to the liposome suspension, and freeze-dry to obtain icarigenin liposomes. The particle size distribution and Zeta potential are shown in Figure 5 , Image 6 .
Embodiment 3
[0046] Weigh 20 mg of icariin, 600 mg of soybean lecithin, and 100 mg of cholesterol, dissolve them in 10 mL of dichloromethane, transfer the dichloromethane solution to a syringe, and slowly inject the dichloromethane solution into 25 mL of 0.5% P-188 (provided by Nanjing Weier Chemical Industry Co., Ltd.) aqueous solution, continue to hydrate for 0.5 hour after the addition, to obtain a liposome suspension. Add 10% mannitol of the suspension volume to the liposome suspension, and freeze-dry to obtain icarigenin liposomes. The particle size distribution and Zeta potential are shown in Figure 7 , Figure 8 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com