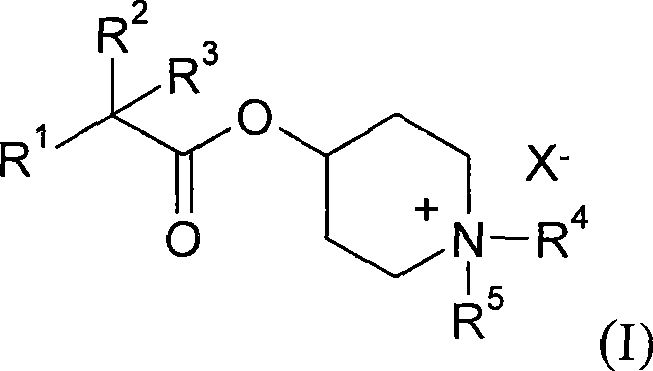
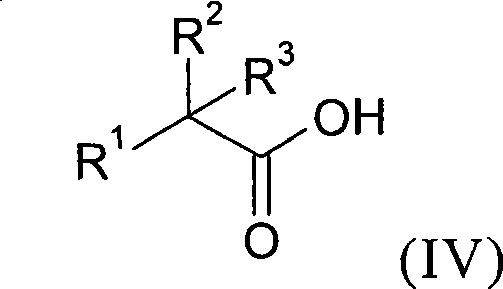
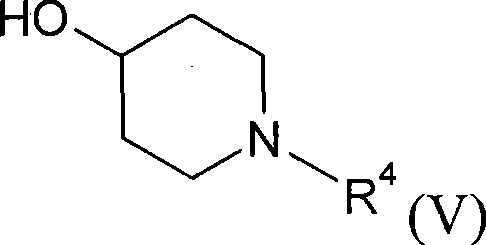
New alkyl esters of cyclic amino alcohols with muscarinic m3 receptor antagonist activity, useful for treating e.g. chronic bronchial obstruction, asthma and overactive bladder
A compound and substituent technology, applied in the field of substituted alkyl ester compounds, can solve the problems of insufficient treatment, short duration, and inconvenience to patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0266] 4-{[(2S)-2-cyclopentyl-2-phenylpropionyl]oxy}-1,1-dimethylpiperidinium iodide
[0267]
[0268] a) Cyclopentyl (phenyl) methyl acetate
[0269]
[0270] A methanol (200 mL) solution of α-phenylcyclopentaneacetic acid (10 g) was treated with trimethylchlorosilane (20 mL), and the resulting mixture was heated under reflux for 3 hours. The solvent was removed under reduced pressure, and the resulting residue was purified by silica gel column chromatography (eluted with 25% diethyl ether in isohexane) to obtain the subtitle compound (10.5 g) as an oil.
[0271] 1 HNMR(400MHz, CDCl 3 )δ 7.35-7.22 (m, 5H), 3.64 (s, 3H), 3.28 (d, 1H), 2.60-2.50 (m, 1H), 1.95-1.85 (m, 1H), 1.70-1.40 (m, 5H) ), 1.30 to 1.20 (m, 1H), 1.03 to 0.97 (m, 1H).
[0272] b) Methyl 2-cyclopentyl-2-phenylpropionate
[0273]
[0274] A lithium diisopropylamide in heptane / tetrahydrofuran / ethylbenzene solution (1.8 molar concentration, 40.1 mL) was added to anhydrous tetrahydrofuran (60 mL), and the result...
Embodiment 2
[0298] 4-{[(2R)-2-cyclopentyl-2-phenylpropionyl]oxy}-1,1-dimethylpiperidinium iodide
[0299]
[0300] a) (4R)-4-benzyl-3-[(2R)-2-cyclopentyl-2-phenylpropionyl]-1,3-oxazolidin-2-one
[0301]
[0302] A toluene (100 mL) solution of 2-cyclopentyl-2-phenyl-propionic acid (the compound prepared in Example 1c, 4.93 g) was treated with thionyl chloride (30 mL), and the resulting mixture was heated at 100°C 2 hours. The solvent was removed under reduced pressure, and the resulting residue was azeotroped with toluene 3 times. The residue was dissolved in anhydrous tetrahydrofuran (20mL), and the resulting mixture was added to -78°C (R)-4-benzyl-2-oxazolone (4.00g) in anhydrous tetrahydrofuran (100mL) in one portion Solution (this solution has been pretreated with a hexane solution of n-butyllithium (1.6 molar concentration, 14.1 mL) at -78°C). The resulting reaction mixture was stirred at -78°C for 30 minutes and then at room temperature for 1 hour. At the end, the mixture was partiti...
Embodiment 3
[0319] 4-[(2-Cyclopropyl-2-phenylpropionyl)oxy]-1,1-dimethylpiperidinium iodide
[0320]
[0321] a) 2-Cyclopropyl-2-phenylpropionic acid (1-methyl-piperidin-4-yl) ester
[0322]
[0323] Treat 2-cyclopropyl-2-phenyl-propionic acid with oxalyl chloride (0.5mL) and N,N-dimethylformamide (20mg) (according to Journal of Organic Chemistry (1989), 54(21), p. Prepared by the method described on pages 5054-63, 110 mg) in dichloromethane (10 mL), and the resulting mixture was stirred at room temperature for 2 hours. The solvent was removed under reduced pressure, and the residue azeotroped with dichloromethane (x3). The resulting residue was dissolved in dichloromethane (2 mL), and then added to a solution of 4-hydroxy-N-methylpiperidine (150 mg) in dichloromethane (2 mL). The resulting reaction mixture was heated at 40°C for 18 hours. The resulting mixture was then partitioned between dichloromethane and aqueous sodium bicarbonate solution, and the organic layer was dried over anhydr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com