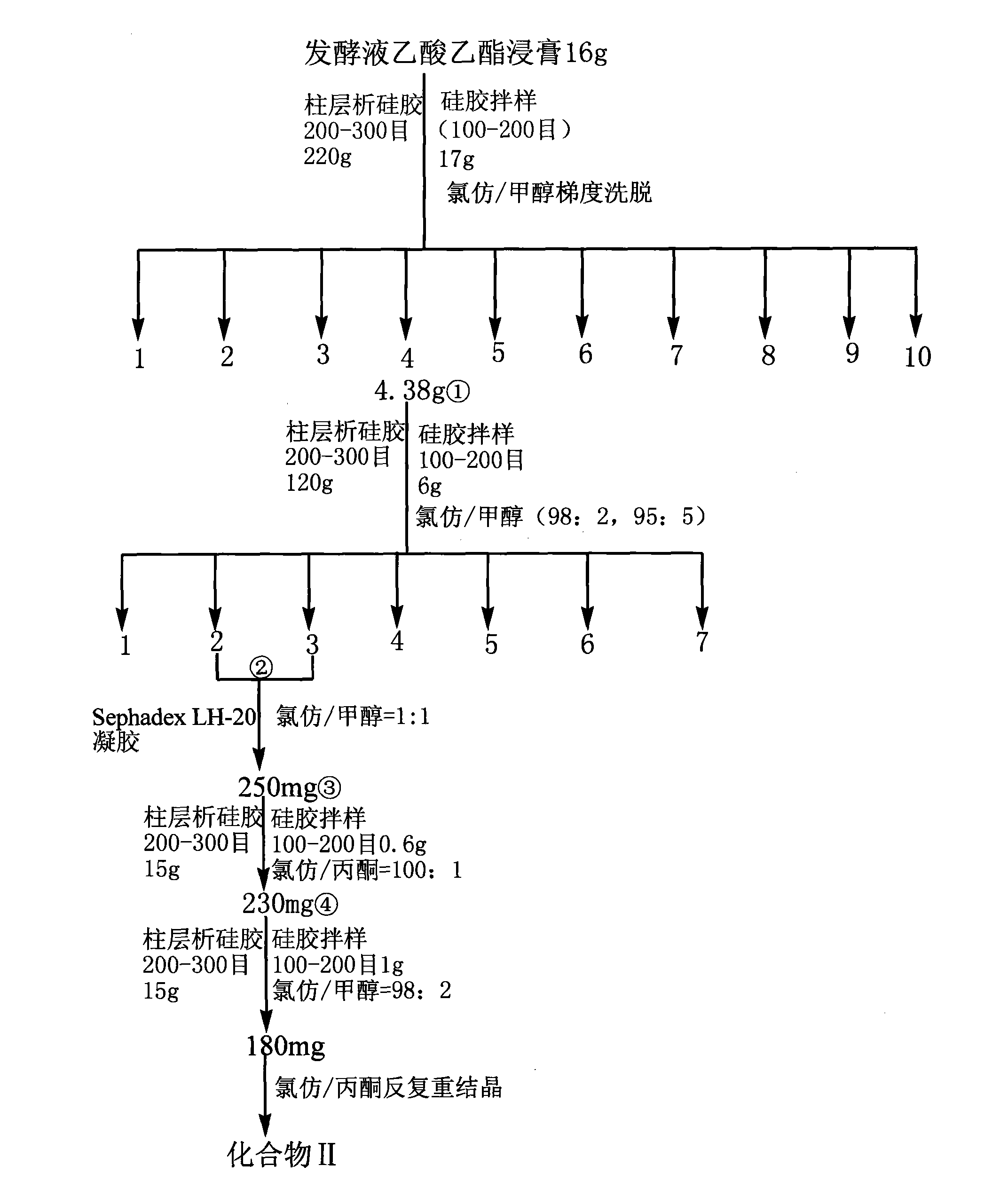
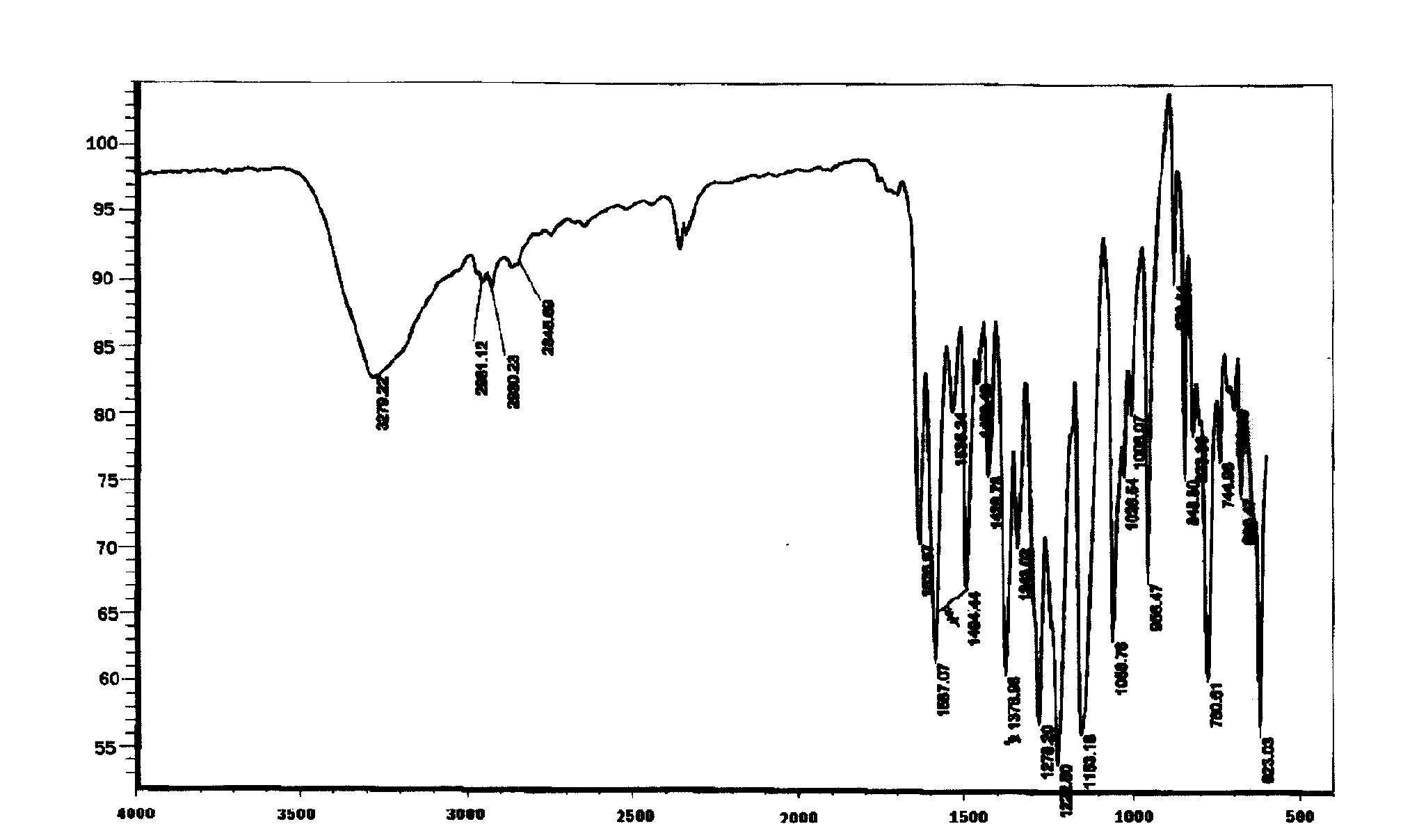
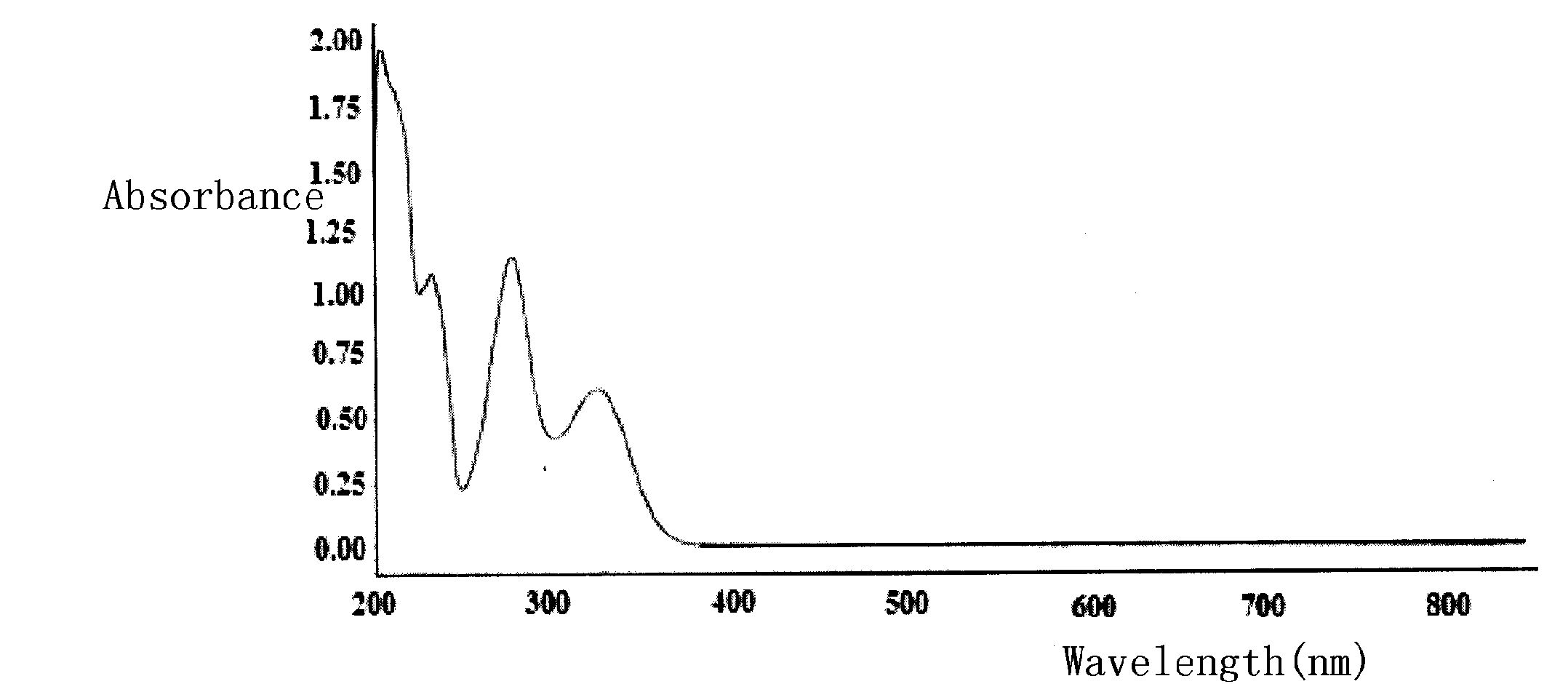
Method for separation preparation of compound 2,4-dihydroxy-5-methyl-acetophenone by using Basidiomycetes
A technology of fermented liquid and porous bacteria, applied in botany equipment and methods, chemicals for biological control, fermentation, etc., can solve the problems of no natural products and anti-phytopathogenic fungal activity, and unclear antibacterial active compounds , to achieve the effects of simple preparation, short fermentation time and mild culture conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0032] This embodiment adopts the method for liquid culture of flat plate spinner bottle, and its concrete steps are as follows:
[0033] 1) Fermentation of bacterial strains and concentration of fermented liquid
[0034] Take 8-10 mm from the activated solid plate bacteria (28°C, cultured for 5-6 days). 2 Insert 2-3 pieces of mushroom cakes of different sizes into the liquid PDA medium made from 200g / L of peeled potatoes, 20g / L of glucose, 1000mL of distilled water, about pH 7.2, and sterilized at 121°C for 30 minutes (the liquid volume is 50mL / 150mL Erlenmeyer flask), at 28°C on a rotary shaker (rotational speed: 150r / min), cultured for 3 to 4 days to form bacterial balls, and then transferred to a fermentation bottle with a 10% inoculation amount (the liquid volume is 150mL / 500mL Erlenmeyer flask), fermented 13d, obtained fermented liquid 50L altogether. Concentrate 50L of fermented broth under reduced pressure at 55°C and halve it;
[0035] 2) Preparation of crude extr...
preparation Embodiment 2
[0044] The present embodiment adopts the method for the liquid culture of plate spinner bottle, and its specific steps are as follows:
[0045] 1) Fermentation of bacterial strains and concentration of fermented liquid
[0046] Take 6~8mm 2 Insert 3 to 4 pieces of mushroom cakes of different sizes into the liquid PDA medium made from 180g / L of peeled potatoes, 18g / L of glucose, 1000mL of distilled water, about pH7.2, and sterilized at 121°C for 30min. 150mL Erlenmeyer flask), cultivated in a rotary shaker (rotating speed: 130r / min) at 25°C for 4 to 5 days to form bacterial balls, and then transferred to a fermentation bottle with a 10% inoculum amount (a liquid volume of 130mL / 500mL) Erlenmeyer flask), fermented 14d, obtained fermented liquid 10L altogether. Concentrate 10L of fermentation broth at 55°C under reduced pressure and reduce it to half;
[0047] 2) Preparation of crude extract
[0048] The concentrated solution of the fermented product was extracted with ethyl ...
preparation Embodiment 3
[0052] The present embodiment adopts the method for the liquid culture of plate spinner bottle, and its specific steps are as follows:
[0053] 1) Fermentation of bacterial strains and concentration of fermented liquid
[0054] Take 6~8mm 2 Insert 2 to 3 pieces of mushroom cakes of different sizes into the liquid PDA medium made of peeled potatoes 250g / L, glucose 25g / L, distilled water 1000mL, pH7. / 150mL Erlenmeyer flask), cultivated in a rotary shaker (170r / min) at 34°C for 3 to 4 days to form bacterial balls, and then transferred to a fermentation flask with a 10% inoculation amount (the liquid volume is 170mL / 500mL Erlenmeyer flask) ), fermented 11d, and obtained a total of 15L of fermented liquid. Concentrate 15L of fermentation broth under reduced pressure at 55°C and reduce it to half;
[0055] 2) Preparation of crude extract
[0056] The concentrated solution of the fermented product was extracted with ethyl acetate, concentrated under reduced pressure to obtain 4....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com