Method for preparing atorvastatin calcium
A technology of atorvastatin calcium and calcium salt, which is applied in the direction of organic chemistry, etc., can solve the problems of low product purity, difficult separation, turbidity, etc., and achieves the effects of easy reaction process, simple reaction steps and stable quality.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1: Preparation of atorvastatin calcium
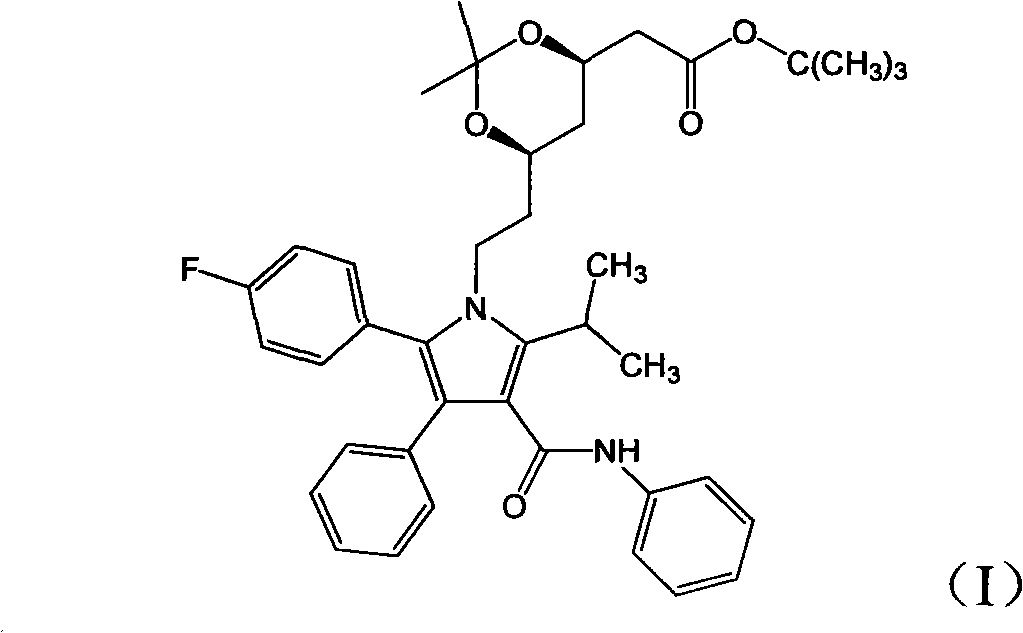
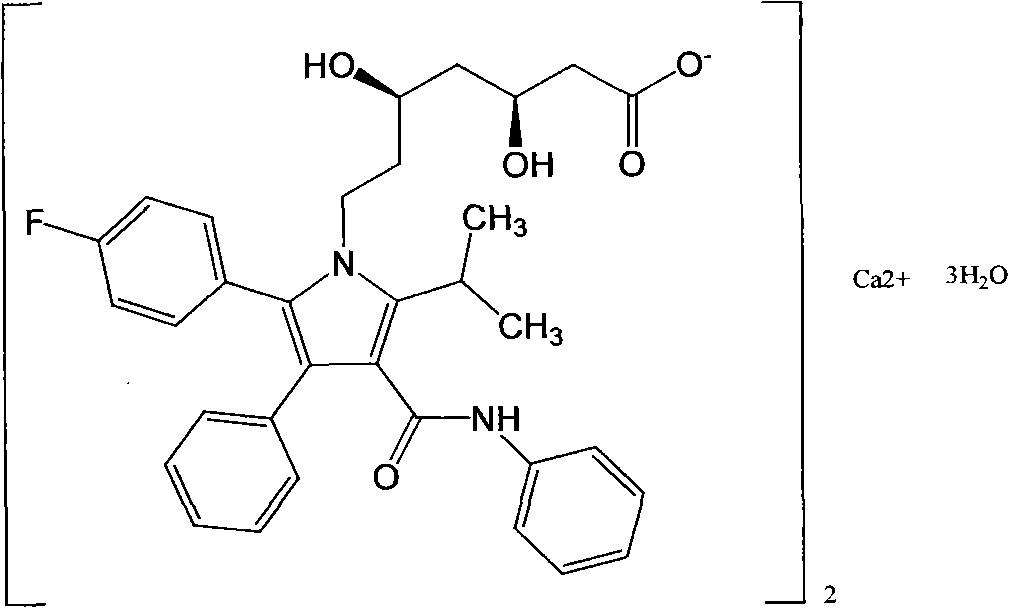
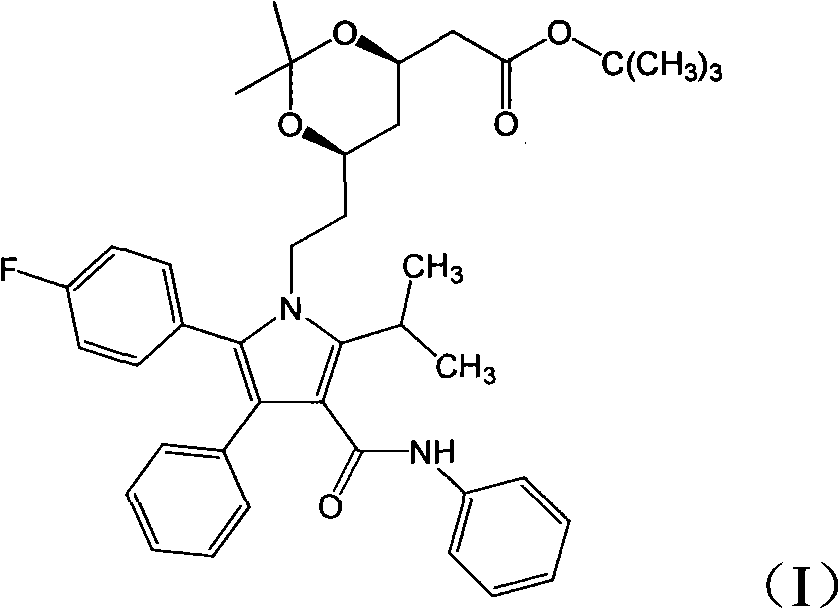
[0025] Add (4R-cis)-6-[2-[2-(4-fluorophenyl)-5-(1-isopropyl)-3-phenyl-4-[(aniline)carbonyl] to the reaction flask -1H-pyrrol-1-yl]ethyl]-2,2-dimethyl-1,3-dioxolane-4-tert-butyl acetate 4g, methanol 30ml, stir to dissolve the solid, add hydrochloric acid 2.4ml , stirred and reacted for 70 minutes; added 0.25g of sodium hydroxide, and reacted for 50 minutes; then added 20ml of methyl tert-butyl ether and 80ml of water, stirred for 10 minutes, separated the phases, and extracted the water phase once with 15ml of methyl tert-butyl ether , the aqueous solution is atorvastatin sodium salt aqueous solution; 12ml aqueous solution of 1.6g of calcium acetate was added to the aqueous solution, reacted for 4 hours, filtered, the filter cake was washed with water, and the obtained solid was vacuum-dried to obtain 3.0g of the final product. Yield 81.3%, HPLC purity 99.1%, [M-Ca+2H] + : 559.6.
Embodiment 2
[0026] Embodiment 2: Preparation of atorvastatin calcium
[0027] Add (4R-cis)-6-[2-[2-(4-fluorophenyl)-5-(1-isopropyl)-3-phenyl-4-[(aniline)carbonyl] to the reaction flask -1H-pyrrol-1-yl]ethyl]-2,2-dimethyl-1,3-dioxolane-4-tert-butyl acetate 4g, methanol 25ml, stir to dissolve the solid, add hydrochloric acid 1.5ml , stirred and reacted for 40 minutes; added 0.5 g of sodium hydroxide, and reacted for 10 minutes; added 20 ml of methyl tert-butyl ether and 60 ml of water, stirred for 10 minutes, separated the phases, and extracted the water phase once with 15 ml of methyl tert-butyl ether, The aqueous solution was the aqueous solution of atorvastatin sodium salt; 1.6 g of calcium acetate in 12 ml of aqueous solution was added to the aqueous solution, reacted for 4 hours, filtered, the filter cake was washed with water, and the obtained solid was vacuum-dried to obtain 3.0 g of the final product. Yield 81.3%, HPLC purity 98.9%, [M-Ca+2H] + : 559.6.
Embodiment 3
[0028] Embodiment 3: Preparation of atorvastatin calcium
[0029] Add (4R-cis)-6-[2-[2-(4-fluorophenyl)-5-(1-isopropyl)-3-phenyl-4-[(aniline)carbonyl] to the reaction flask -1H-pyrrol-1-yl]ethyl]-2,2-dimethyl-1,3-dioxolane-4-tert-butyl acetate 4g, ethanol 30ml, stir to dissolve the solid, add hydrochloric acid 2.4ml , stirred and reacted for 20 minutes; added potassium hydroxide 1.7g, reacted for 5 minutes; added 20ml methyl tert-butyl ether and 210ml water, stirred for 10 minutes, separated the phases, and extracted the water phase once with 15ml methyl tert-butyl ether, The aqueous solution was atorvastatin sodium salt aqueous solution; 1.6 g of calcium acetate in 12 ml aqueous solution was added to the aqueous solution, reacted for 4 hours, filtered, the filter cake was washed with water, and the obtained solid was vacuum-dried to obtain 2.8 g of the final product. Yield 75.9%, HPLC purity 99.1%, [M-Ca+2H] + : 559.6.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com