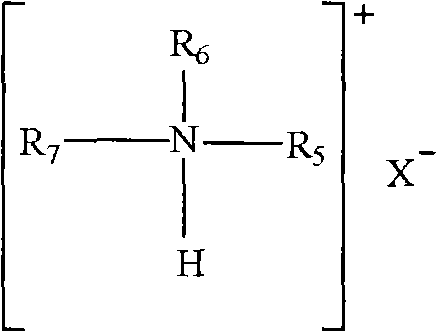
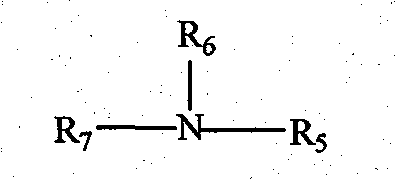Method for preparing liquid containing metallic ions by employing extraction reaction
A metal ion, liquid technology, used in the preparation of amino compounds from amines, bulk chemical production, organic chemistry, etc., to achieve the effects of reducing environmental pollution, short time, and low energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] (1) Take 10mL N235 and 30mL 0.84mol / L hydrochloric acid solution in a 60mL separatory funnel, shake at room temperature for 10min, centrifuge to separate the phases, and take the upper phase, which is the [N235H]Cl intermediate.
[0052] Take 10mL [N235H]Cl intermediate and 30mL ferric chloride solution, C Fe 3+ =38.3265mg / mL, hydrochloric acid concentration C HCl =3.6339mol / L, Fe 3+ The ions and [N235H]Cl were mixed in a 60mL separatory funnel at a molar ratio of 1:1, shaken at a constant temperature of 20°C for 90 minutes, centrifuged to separate the phases, and the upper phase was taken, which was dark brown [NR 3 H][FeCl 4 ] ionic liquid with a yield of 54.83%.
[0053] (2) [NR prepared by (1) 3 H][FeCl 4] The ionic liquid and petroleum ether were mixed at a volume ratio of 1:2, oscillated for 3 minutes, and the phases were separated at rest. The collected ionic liquid solvent was evaporated, and vacuum-dried at 70°C for 24h to obtain dark brown [NR 3 H][FeC...
Embodiment 2
[0055] (1) Take 10mL N235 and 30mL 1.2mol / L hydrochloric acid solution in a 60mL separatory funnel, shake at room temperature for 30min, centrifuge to separate the phases, and take the upper phase, which is the [N235H]Cl intermediate.
[0056] Take 10mL [N235H]Cl intermediate and 30mL ferric chloride solution, C Fe 3+ =38.3265mg / mL, hydrochloric acid concentration C HCl =1.5989mol / L, Fe 3+ The moles of ions and [N235H]Cl were mixed in a 60mL separatory funnel at a ratio of 1:1, shaken at a constant temperature of 25°C for 30min, centrifuged to separate the phases, and the upper phase was taken. The upper phase is dark brown [NR 3 H][FeCl 4 ] ionic liquid, the yield is 46.01%.
[0057] (2) [NR prepared by (1) 3 H][FeCl 4 ] The ionic liquid and petroleum ether were mixed at a volume ratio of 1:2, oscillated for 10 min, and the phases were separated at rest, and the lower phase was rich in ionic liquid; the rich ionic liquid in the lower phase was separated; the above step...
Embodiment 3
[0060] (1) Take 10mL N235 and 30mL 0.96mol / L hydrochloric acid solution in a 60mL separatory funnel, shake at room temperature for 20min, centrifuge to separate the phases, and take the upper phase, which is the [N235H]Cl intermediate.
[0061] Take 8mL [N235H]Cl intermediate and 24mL cupric chloride solution, C Cu 2+ =43.4592mg / mL, hydrochloric acid concentration C HCl =2.3257mol / L, Cu 2+ The moles of ions and [N235H]Cl were mixed in a 60mL separatory funnel at a ratio of 1:1, shaken at a constant temperature of 38°C for 120min, centrifuged to separate the phases, and the upper phase was taken. The upper phase is yellow [NR 3 H] 2 [CuCl 4 ] ionic liquid, the yield is: 21.85%.
[0062] (2) [NR prepared by (1) 3 H] 2 [CuCl 4 ] The ionic liquid and petroleum ether were mixed at a volume ratio of 1:2, oscillating for 8 minutes, and the phases were separated at rest. The collected ionic liquid solvent was evaporated and dried in vacuum at 70°C for 18h to obtain a yellow ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com