Photosensitive resin composition, cured product thereof, and method for producing photosensitive resin
A technology of photosensitive resin and composition, which is applied in the field of photosensitive resin composition, can solve problems such as insufficient satisfaction, and achieve the effects of excellent flame retardancy and excellent photosensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
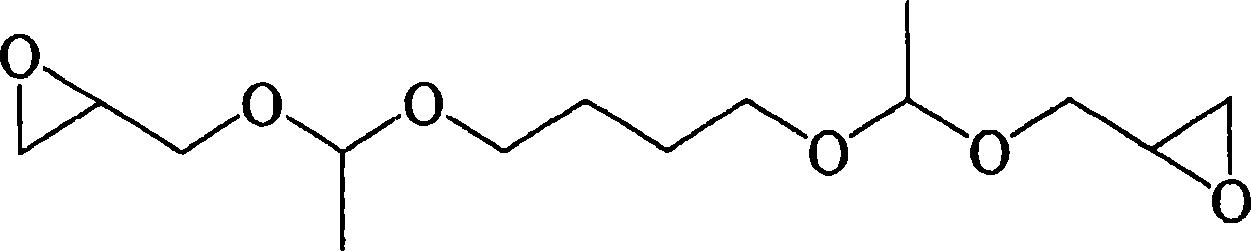
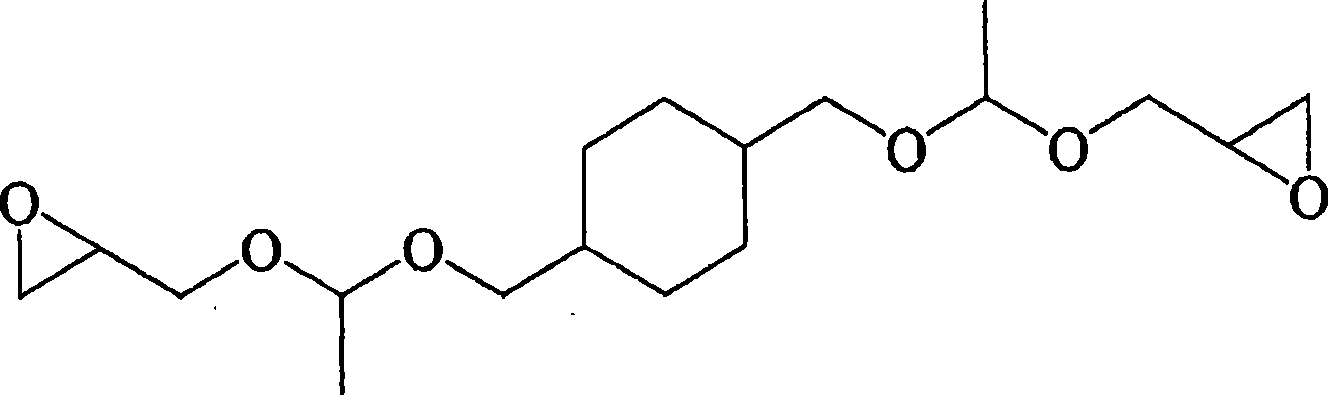
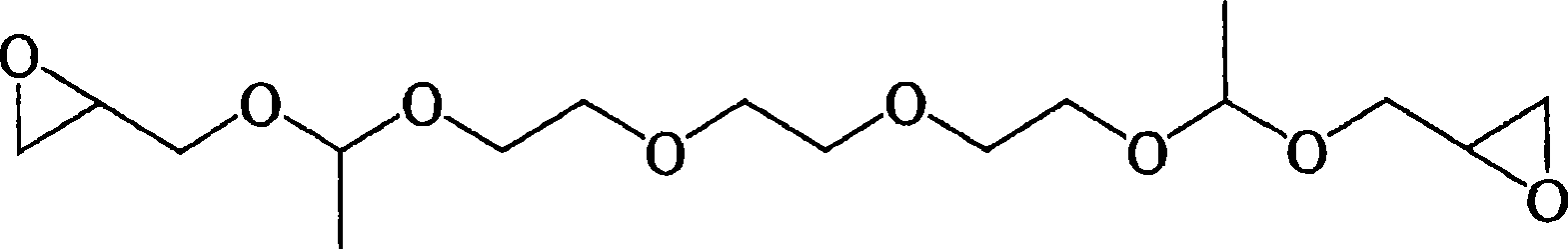
Image
Examples
preparation example Construction
[0106] The preparation method provided by the present invention of the carboxyl-containing photosensitive polyurethane resin (A) includes:
[0107] The first step in which the polymer polyol (e), the carboxylic acid compound (f) and the diisocyanate compound (g) having two hydroxyl groups in the molecule are reacted as essential components to obtain a carboxyl-containing polyurethane prepolymer (a),
[0108] The carboxyl-containing polyurethane prepolymer (a) obtained in the first step reacts with a compound (b) having an epoxy group or an oxetanyl group and an ethylenically unsaturated group to obtain a hydroxyl-containing polyurethane prepolymer the second step of (c), and
[0109] A third step of reacting the hydroxyl group-containing polyurethane prepolymer (c) obtained in the second step with the acid anhydride group-containing compound (d).
[0110] As the reaction conditions of the first step, it is preferable to react by heating and stirring at a temperature of room t...
Embodiment
[0206] The following examples illustrate the present invention more specifically, but the following examples do not limit the scope of rights of the present invention in any way. In addition, "part" in an Example means a "weight part."
[0207] The measurement conditions of GPC are as follows.
[0208]
[0209] For the measurement of Mw, GPC (gel permeation chromatography) "HPC-8020" manufactured by Tosoh Corporation was used. GPC is a liquid chromatography that separates and quantifies substances dissolved in a solvent (THF:tetrahydrofuran) based on the difference in molecular size. In the measurement in the present invention, two columns of "LF-604" (manufactured by Showa Denko Co., Ltd.: GPC column for rapid analysis: 6mmID x 150mm size) are used in series, at flow rate 0.6ml / min, column temperature It carried out on the condition of 40 degreeC, and the determination of the weight average molecular weight (Mw) was carried out by polystyrene conversion.
preparation example 1
[0211] Add polytetramethylene glycol (PTG1000sn: manufactured by Hodo Ketani Chemical Co., Ltd.: hydroxyl value=110mgKOH / g, Mw=1020) into a four-necked flask equipped with a stirrer, reflux cooling pipe, nitrogen gas introduction pipe, introduction pipe, and thermometer 156 129 parts, 129 parts of dimethylolbutyric acid (manufactured by Nippon Chemicals Co., Ltd.), and 375 parts of cyclohexanone as a solvent were heated up to 60° C. while stirring under a nitrogen stream, and dissolved uniformly. Next, 215 parts of isophorone diisocyanate was put into this flask, and it stirred at 90 degreeC for 8 hours, and performed the urethanization reaction. After completion of the reaction, a small amount of sampling was performed to obtain a carboxyl group-containing polyurethane prepolymer having a polystyrene-equivalent weight average molecular weight of 13,000 and an actually measured acid value of the resin solid content of 98 mgKOH / g.
[0212] Then, the introduction of nitrogen gas...
PUM
| Property | Measurement | Unit |
|---|---|---|
| acid value | aaaaa | aaaaa |
| acid value | aaaaa | aaaaa |
| acid value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com