Eyedrop preparation and preparation method thereof
A preparation and eye instillation technology, applied in the field of medicine, can solve the problems of blurred vision and poor patient compliance, and achieve the effects of improving drug stability, facilitating medication, and improving local bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The eye drop preparation described in this embodiment is an eye drop solution, based on 100 g, containing 0.10 g of doxycycline, 1.00 g of hydroxypropyl-β-cyclodextrin, and 0.10 g of sodium bisulfite, pH 5.5-6.0 .
[0033] Preparation method: Take doxycycline hydrochloride (containing 0.10g of doxycycline), 1.00g of hydroxypropyl-β-cyclodextrin, add a small amount of distilled water until moist, fully grind in a mortar for 3 hours, add an appropriate amount of distilled water to dilute, Obtain light yellow clear doxycycline clathrate solution, add sodium bisulfite 0.10g, add distilled water to 100g, adjust the pH value in the process to be 5.0-6.0 (pH regulator can be disodium hydrogen phosphate, dihydrogen phosphate sodium, sodium hydroxide, or sodium bicarbonate), membrane filter sterilization, doxycycline eye drops.
Embodiment 2
[0035] The eye drop preparation described in this embodiment is an eye drop solution, based on 100 g, containing 0.10 g of doxycycline, 1.00 g of hydroxypropyl-β-cyclodextrin, and 0.10 g of sodium bisulfite, pH 5.5-6.0 .
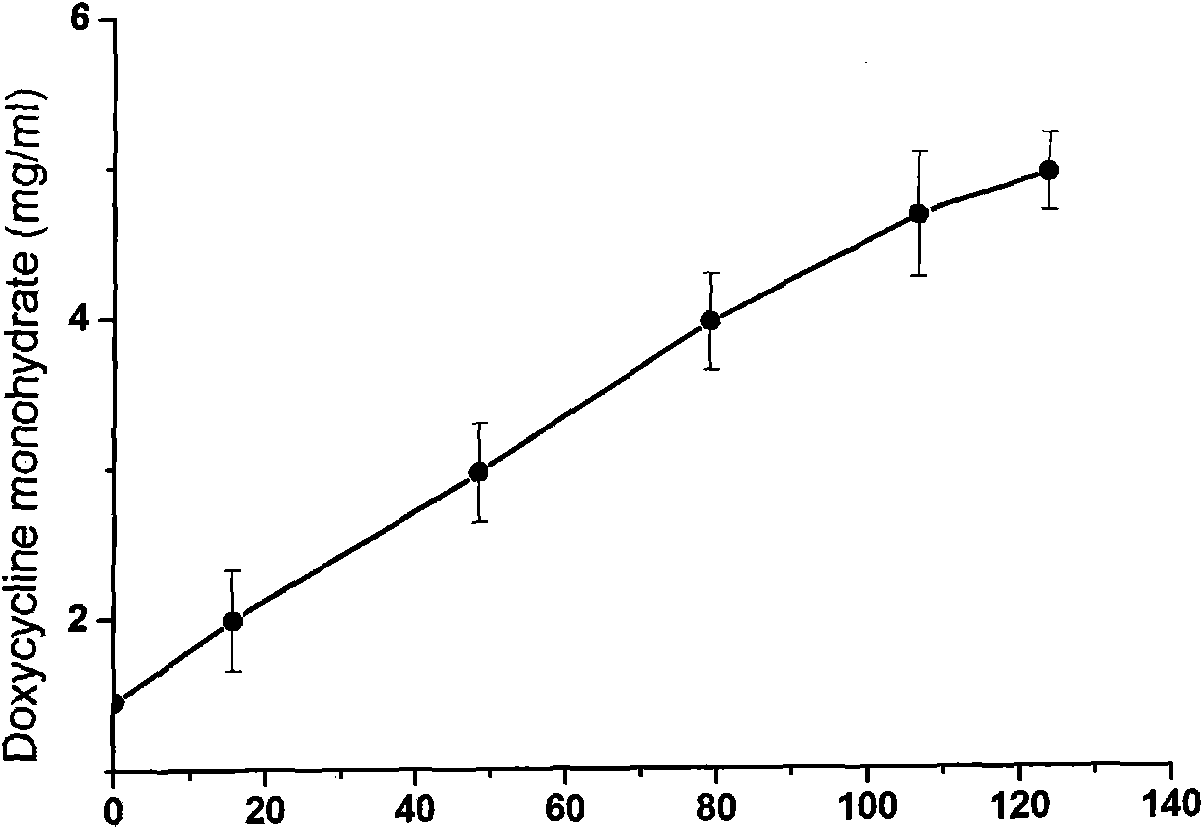
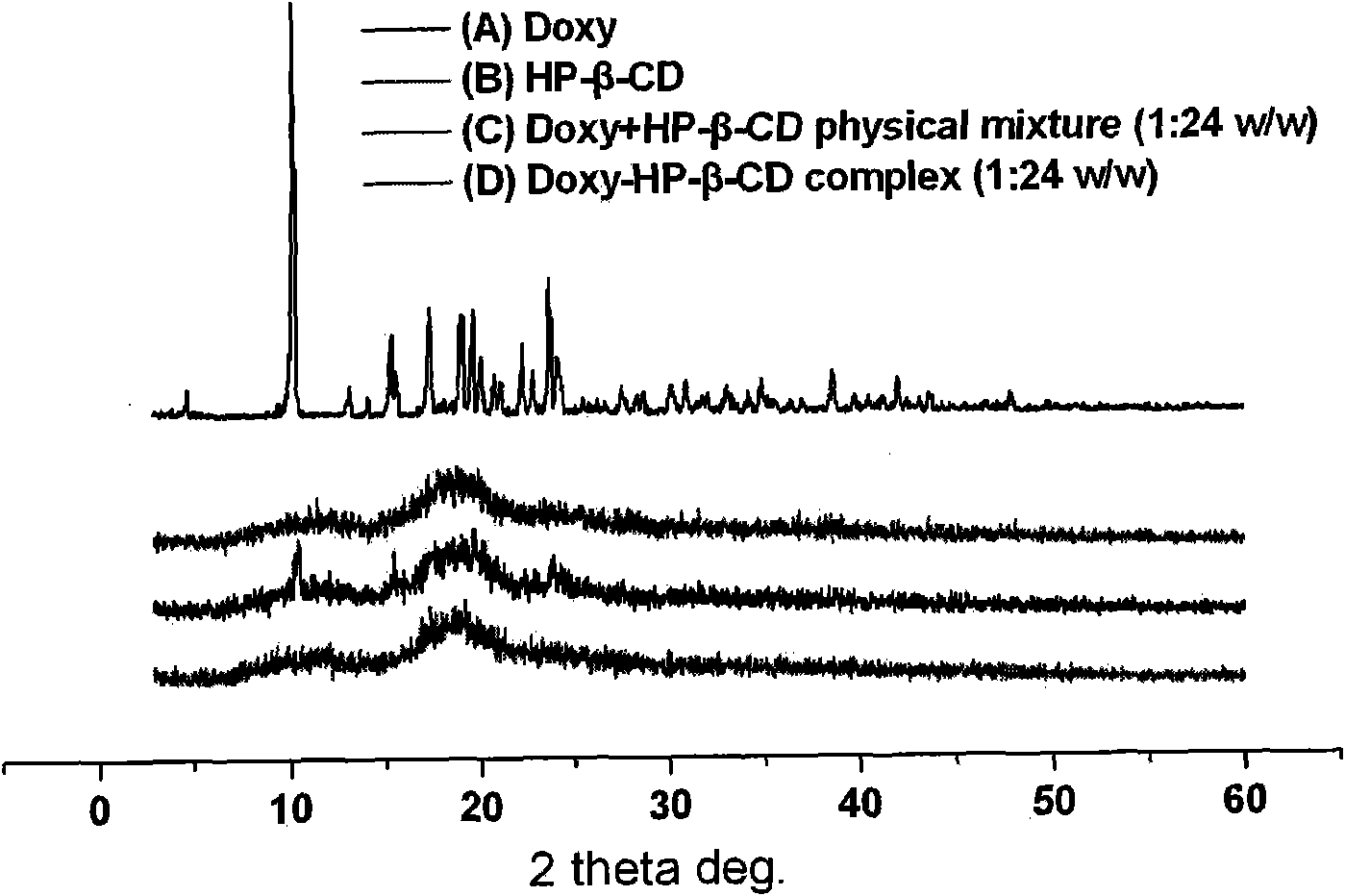
[0036] Preparation method: Take 1.00 g of hydroxypropyl-β-cyclodextrin and dissolve it in 10 g of distilled water to obtain a solution of hydroxypropyl-β-cyclodextrin; take doxycycline (containing 0.10 g of doxycycline monohydrate ), dispersed in about 5.0 g of distilled water, slowly added dropwise to the hydroxypropyl-β-cyclodextrin solution while stirring, and ultrasonicated for 3 hours to obtain a pale yellow clear doxycycline inclusion compound solution. By phase dissolution and X-ray diffraction analysis, as figure 1 and figure 2 . in, figure 1 (A) doxycycline monohydrate (B) hydroxypropyl-β-cyclodextrin (C) physical mixture of doxycycline monohydrate and hydroxypropyl-β-cyclodextrin (1:24 w / w) (D) Doxycycline monohydrate hydroxypropyl-β-cyclode...
Embodiment 3
[0040] The eye drop preparation described in this example is a temperature-sensitive in-situ gel eye drop, containing 0.03 g of doxycycline, 0.90 g of hydroxypropyl-β-cyclodextrin, and poloxamer 407 in 100 g. 16.00g, sodium bisulfite 0.10g, pH 5.5-6.0.
[0041] Preparation method: Take 0.90 g of hydroxypropyl-β-cyclodextrin, dissolve it in 10 g of distilled water to obtain a solution of hydroxypropyl-β-cyclodextrin; take doxycycline (containing 0.03 g of doxycycline monohydrate ), dispersed in about 1.5 g of distilled water, slowly added dropwise to the hydroxypropyl-β-cyclodextrin solution while stirring, and ultrasonicated for 3 hours to obtain a light yellow clear doxycycline inclusion complex solution, which was solution A.
[0042] Take 16.00g of Poloxamer 407, slowly disperse it into 50g of distilled water, and store it in cold storage for 12-24h until a clear and uniform solution is formed, which is solution B.
[0043] Fully mix the above solution A with solution B, a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| phase transition temperature | aaaaa | aaaaa |
| phase transition temperature | aaaaa | aaaaa |
| phase transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com