Preparing method of 2-methoxy imino group 2-furan ammonium acetate
A technology of methoxyimino and amine furanoacetate is applied in the field of preparation of 2-methoxyimino-2-furanoacetate amine, and can solve the problems of many types of solvents, large amount of by-product furoic acid, large solvent consumption and the like , to reduce costs and improve yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0019] A detailed description will be given below of specific embodiments of the present invention.
[0020] The preparation method of 2-methoxyimino 2-furan acetate amine, the method comprises the following steps:
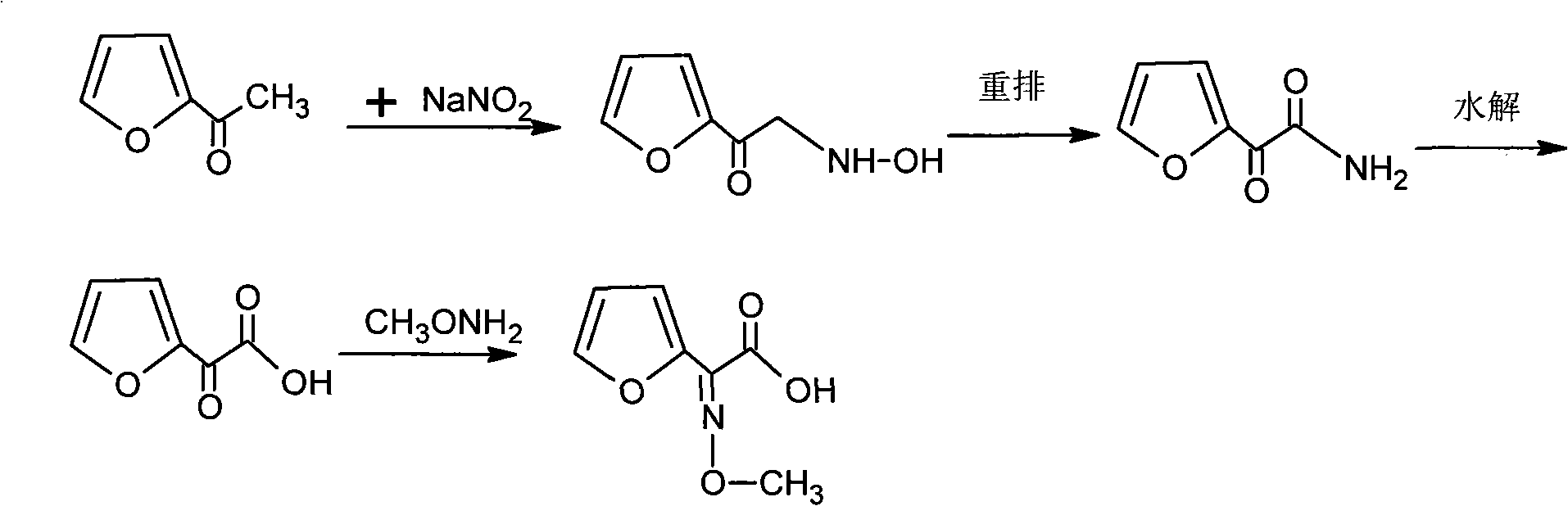
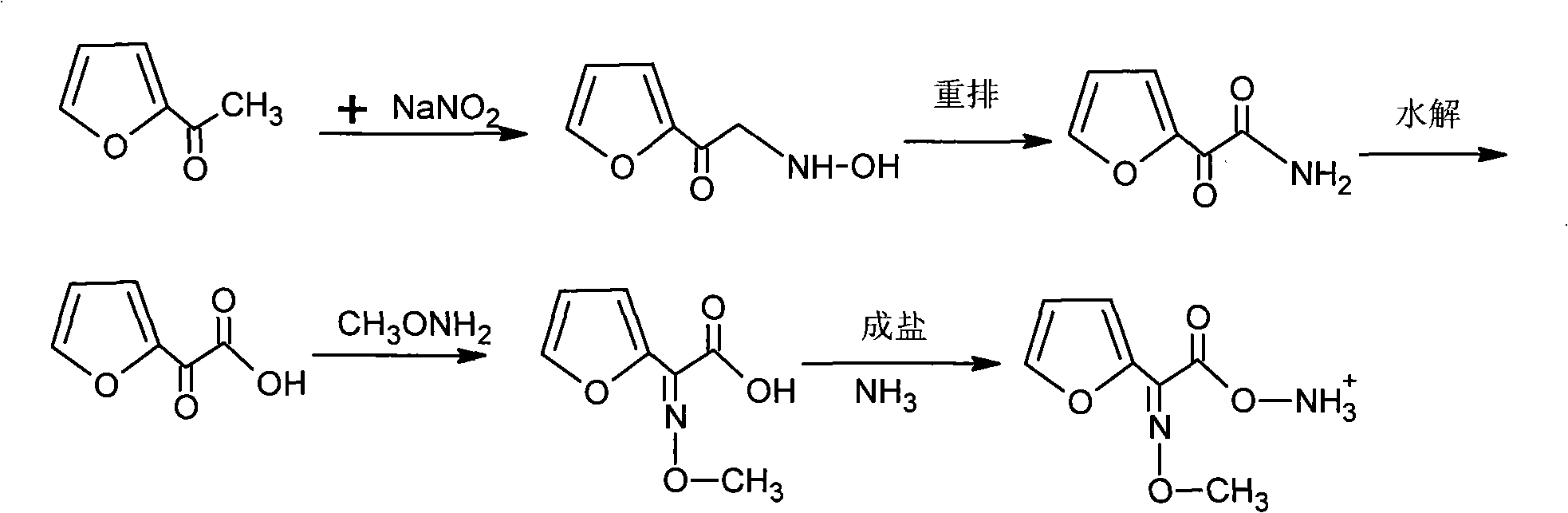
[0021] ①. Reaction of sodium nitrite and furan acetate, the reaction temperature is 59°C±1°C, and furanonic acid is generated through oximation, rearrangement, and hydrolysis;
[0022] ②, add hydrochloric acid, adjust the pH to 0.2, use butyl acetate to extract, then add Na 2 CO 3 Solution stripping obtains furanone acid;
[0023] ③. Condensation of furanone acid and methoxyammonium hydrochloride to generate 2-imino 2-furan acetic acid;
[0024] ④, adjust the pH to 0.5, use butyl acetate to extract 2-imino-2-furanacetic acid, add activated carbon for dehydration and decolorization;
[0025] ⑤. Add methanol with solvent volume of 5-10% in the reaction solution, and then pass ammonia gas to form a salt to generate 2-methoxyimino 2-furanacetate amine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com