Phenyl propanoid derivative, preparation method thereof and application thereof to preparation of medicines resisting breast cancer
A derivative, phenylpropanoid technology, applied in the application field of phenylpropanoid derivatives and its preparation, preparation of anti-breast cancer drugs, achieving high purity, accurate results, and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
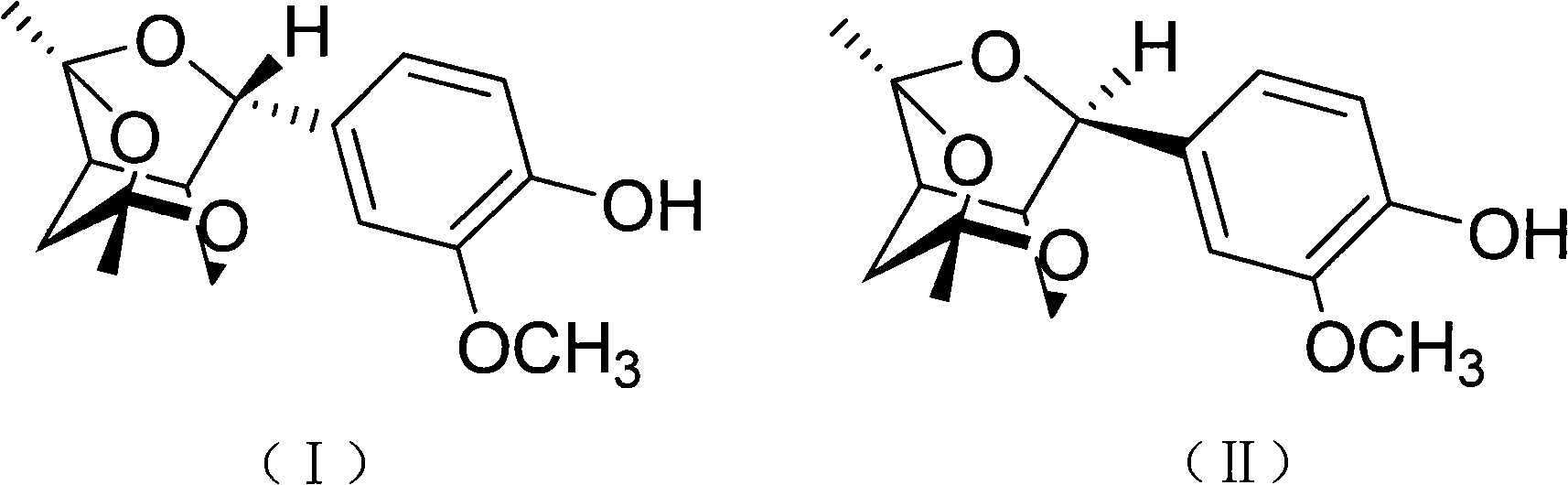
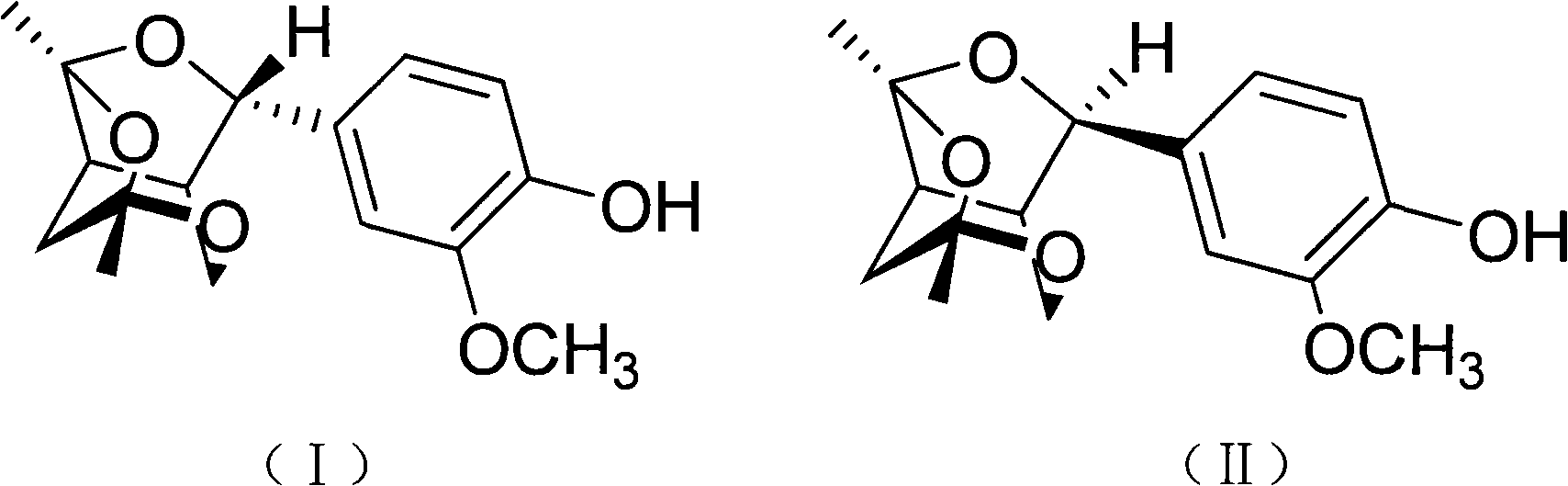
[0032] Preparation of compounds shown in embodiment 1 formula (I) and formula (II)
[0033] In this example, the extraction, extraction, column chromatography and thin layer chromatography commonly used by those skilled in the art to obtain a certain active ingredient from plants were used to obtain two new species from the stems and branches of the mangrove plant pomegranate. substances, and carried out spectral signal analysis and identification on them, and found that the structural formulas of these two substances are shown in formula (I) and formula (II), these two compounds are a pair of epimers, belonging to phenylpropanoid compounds , but has a new skeleton structure different from existing phenylpropanoids.
[0034] The preparation method of the present embodiment, its specific steps are as follows:
[0035] (1) The stems and branches of the mangrove plant pomegranate are naturally dried and crushed, and 4.9Kg of the crushed material is taken, soaked and extracted at...
Embodiment 2
[0050] Lethal activity experiment of embodiment 2Catunaregin and Epicatunaregin to saltwater shrimp
[0051] Take a 96-well cell culture plate and add 200 μl of artificial seawater containing 10-15 sea shrimp larvae to each well to make a test culture plate.
[0052] This embodiment establishes a blank control group and a sample group: the blank control group is to add 5 μ l of solvent dimethyl sulfoxide (DMSO) to the above-mentioned test culture plate; the sample group is prepared by dissolving the obtained Catunaregin prepared in Example 1 with the solvent DMSO Catunaregin content is 500μg / mL, 50μg / mL and 5μg / mL three concentrations of sample solution, three groups of sample components, 1 group is to add 5μl concentration of 500μg / mL sample solution to the above test culture plate, 2 groups Add 5 μl of sample solution with a concentration of 50 μg / mL to the above-mentioned test culture plate, and add 5 μl of sample solution with a concentration of 5 μg / mL to the above-mentio...
Embodiment 3
[0060] Embodiment 3MTT method measures the antitumor activity of Catunaregin and Epicatunaregin
[0061] In this example, the tumor cell-human breast cancer F10 (commercially available) was selected as the research object, and the tumor cell growth inhibition experiment was carried out on Catunaregin and Epicatunaregin by using the MTT colorimetric method routinely used by those skilled in the art to detect cell survival and growth.
[0062] Sample group: Catunaregin prepared in Example 1 was dissolved in DMSO to prepare five sample groups with Catunaregin content of 0.01, 0.1, 1, 10 and 100 μg / mL.
[0063] Positive control group: 5-fluorouracil.
[0064] Negative control group: complete cell culture medium with 0.5 volume % DMSO (commercially available).
[0065] F10 cells in the logarithmic growth phase were collected and seeded in 96-well culture plates, with the number of cells per well being 1.0×10 5 / 100μl, placed in 5vol% CO 2 Culture in an incubator, remove the medi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com