Method for establishing snake poison fingerprint maps and fingerprint maps thereof
A technique for fingerprinting and establishing methods, which is applied in measuring devices, instruments, scientific instruments, etc., can solve the problems of unseen popularization and application, limited small molecules, etc., and achieve the effects of easy mastery, high precision, and simple methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
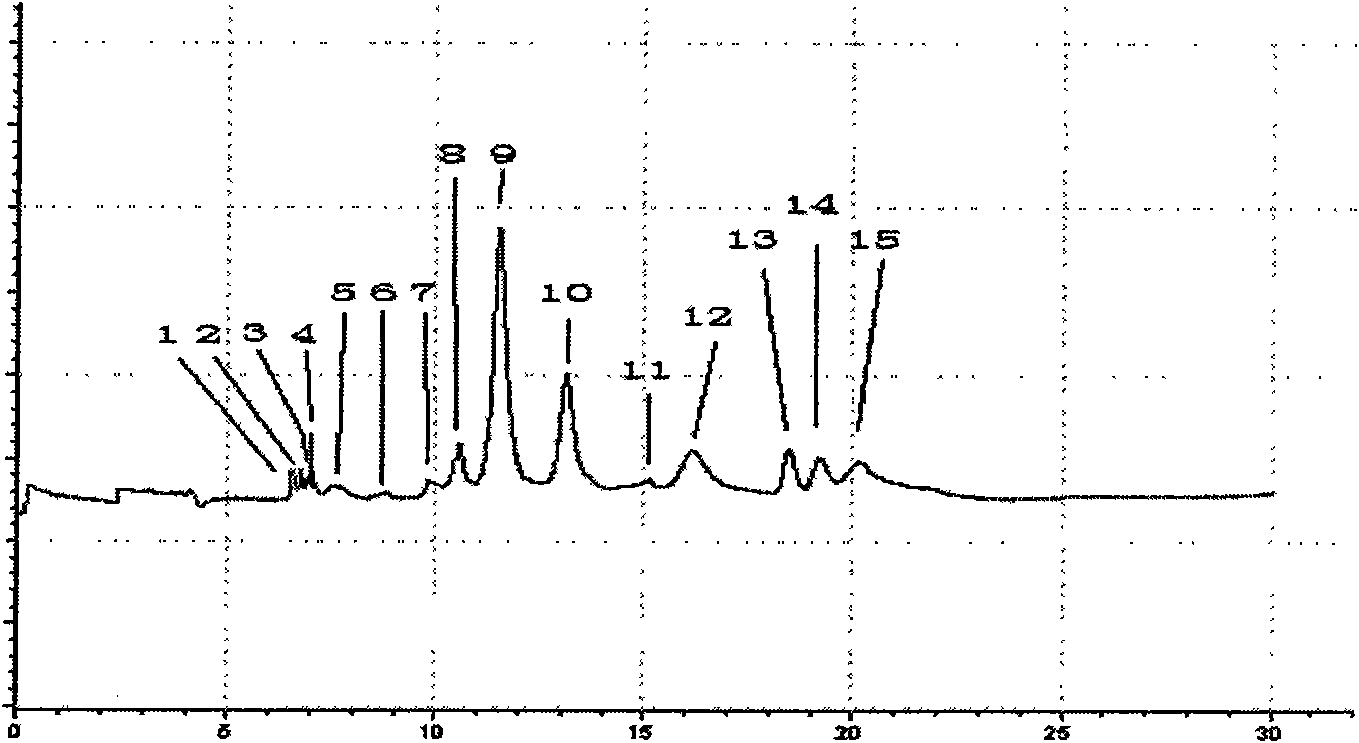
[0042] 1 Preparation of test solution and reference solution
[0043] Take 11 batches of Agkistrodon Agkistrodon venom, mix them uniformly, dissolve them in 10ml of distilled water, mix them by ultrasonic vibration, centrifuge at a high speed (10000rpm, 10min), and take the supernatant to obtain the test solution. Ready to use.
[0044] Accurately weigh 10 mg of the plasmin reference substance, dissolve it in 1 ml of distilled water as the reference substance solution;
[0045] 2 Electrophoresis conditions
[0046] The uncoated capillary is 75μm×35cm, the effective length is 25cm (Hebei Yongnian Optical Fiber Factory); the ultraviolet detection wavelength is 195nm; the operating voltage is 10kV; the capillary temperature is 18°C; the sampling method is 0.5psi, 5s. Before using a new capillary column, wash the capillary column with methanol, 1mol / L HCl, 1mol / L NaOH, and pure water for 10 minutes each. The capillary column was rinsed with 0.1mol / L NaOH and purified water for ...
Embodiment 2
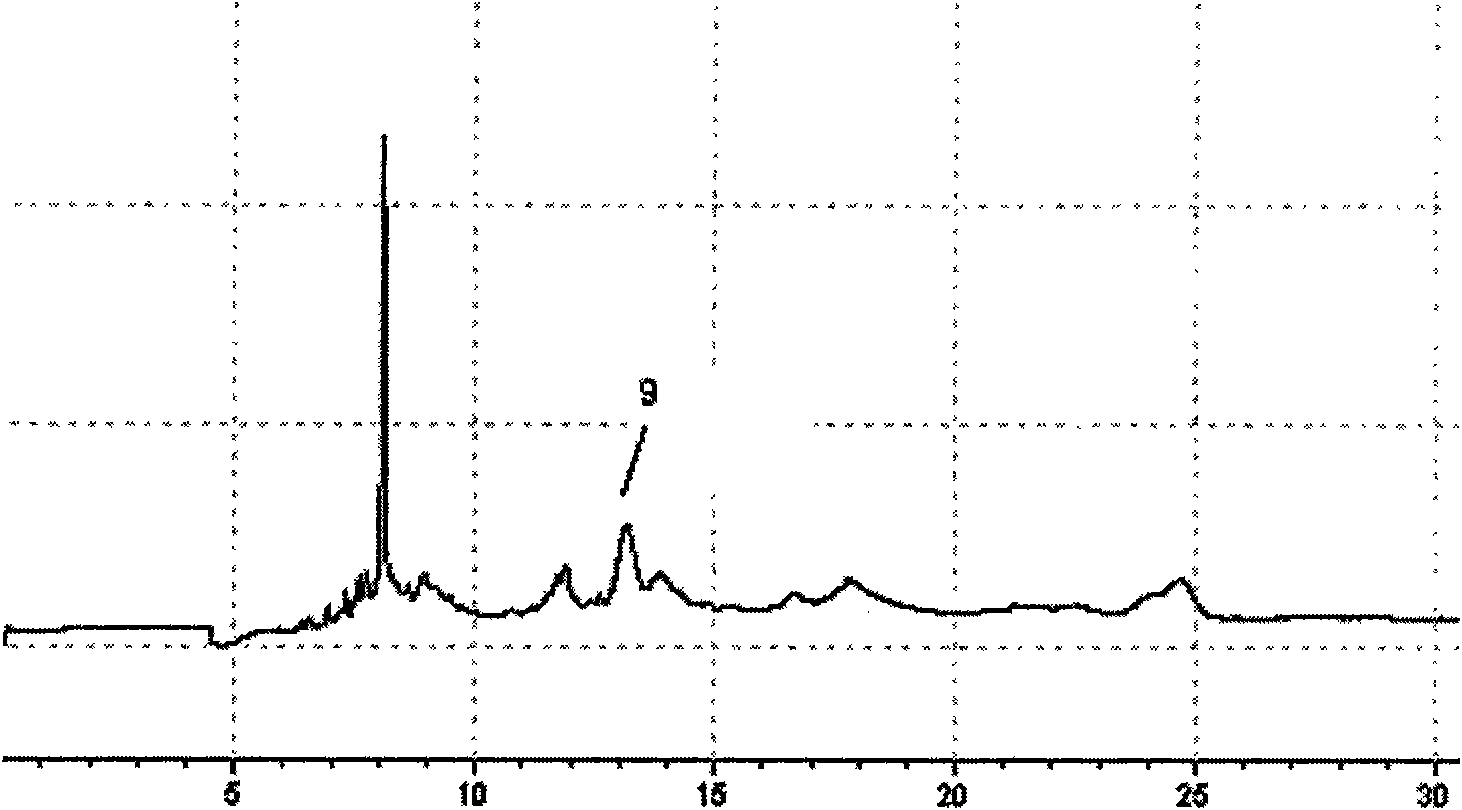
[0056] 1 Preparation of the test solution
[0057] Take 11 batches of Agkistrodon Agkistrodon venom, mix them uniformly, dissolve them in 10ml of distilled water, mix them by ultrasonic vibration, centrifuge at a high speed (10000rpm, 10min), and take the supernatant to obtain the test solution. Ready to use.
[0058] Accurately weigh 10 mg of the plasmin reference substance, dissolve it in 1 ml of distilled water as the reference substance solution;
[0059] 2 Electrophoresis conditions
[0060] The uncoated capillary is 75μm×35cm, the effective length is 25cm (Hebei Yongnian Optical Fiber Factory); the ultraviolet detection wavelength is 195nm; the operating voltage is 10kV; the capillary temperature is 18°C; the sampling method is 0.5psi, 5s. Before using a new capillary column, wash the capillary column with methanol, 1mol / L HCl, 1mol / L NaOH, and pure water for 10 minutes each. The capillary column was rinsed with 0.1mol / L NaOH and purified water for 10 min before and a...
Embodiment 3
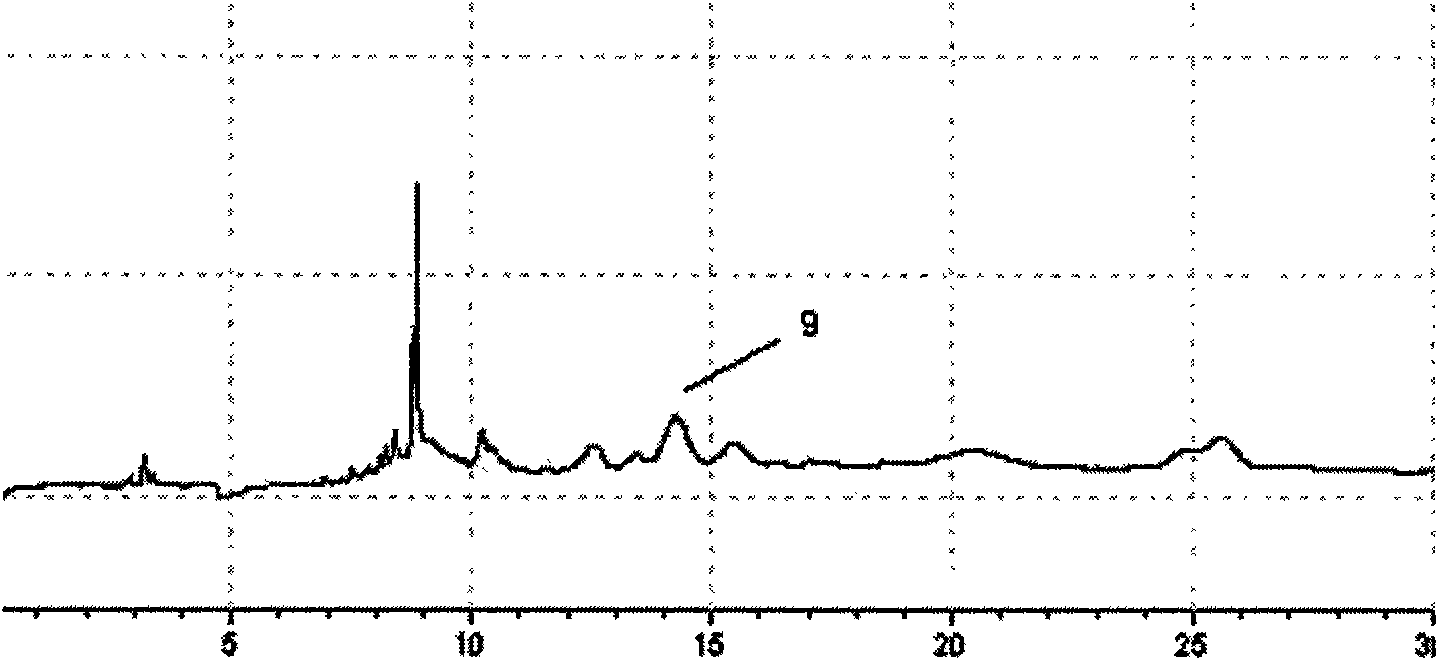
[0071] 1 Preparation of the test solution
[0072] Take 11 batches of Agkistrodon Agkistrodon venom, mix them uniformly, dissolve them in 10ml of distilled water, mix them by ultrasonic vibration, centrifuge at a high speed (10000rpm, 10min), and take the supernatant to obtain the test solution. Ready to use.
[0073] Accurately weigh 10 mg of the plasmin reference substance, dissolve it in 1 ml of distilled water as the reference substance solution;
[0074] 2 Electrophoresis conditions
[0075] The uncoated capillary is 75μm×35cm, the effective length is 25cm (Hebei Yongnian Optical Fiber Factory); the ultraviolet detection wavelength is 195nm; the operating voltage is 10kV; the capillary temperature is 18°C; the sampling method is 0.5psi, 5s. Before using a new capillary column, wash the capillary column with methanol, 1mol / L HCl, 1mol / L NaOH, and pure water for 10 minutes each. The capillary column was rinsed with 0.1mol / L NaOH and purified water for 10 min before and a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com