Compound containing pigment epidermal derived factors and preparation method and application thereof
A technology of deriving factors and pigment epithelium, which is applied in the direction of non-active ingredients of polymer compounds, medical preparations containing active ingredients, biological testing, etc., can solve the problems of patient inconvenience, increased drug costs, and short half-life, and achieve high inhibition and small Effects of mouse tumor growth, slowing metabolism, and prolonging half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0043] The preparation method of the complex containing pigment epithelium-derived factor of the present invention comprises the following steps:
[0044] The activated polyenol compound is mixed with the pigment epithelium-derived factor at a molar ratio of 1:5 to 5:1, and reacted at a pH of 3-10 and a temperature of 4-30°C. Preferably, the polyenol compound is polyethylene glycol.
[0045] Preferably, different modified pigment epithelium-derived factors obtained after the reaction are separated by a cationic column or molecular sieve to obtain a complex of PEG modified at a single site at the N-terminal of the pigment epithelium-derived factor.
[0046] The present invention also provides a slow-release preparation comprising the pigment epithelium-derived factor complex, and the slow-release material of the slow-release preparation is selected from microcapsules, hydrogels, microspheres, micro-osmotic pumps or liposomes. The sustained-release preparation can further prolo...
Embodiment 1
[0048] Example 1 Preparation of recombinant pigment epithelium-derived factor
[0049] (1) Construction of strains
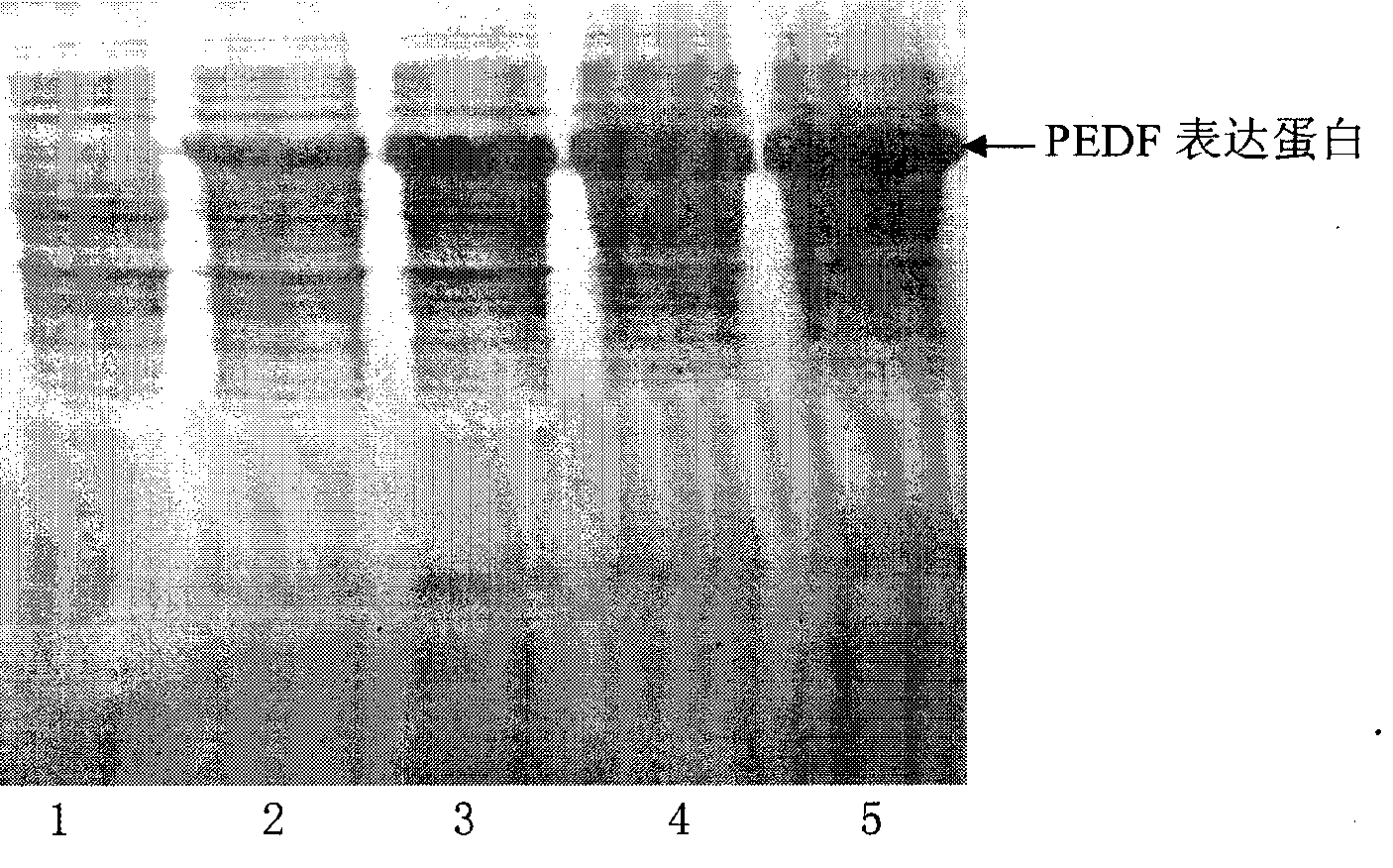
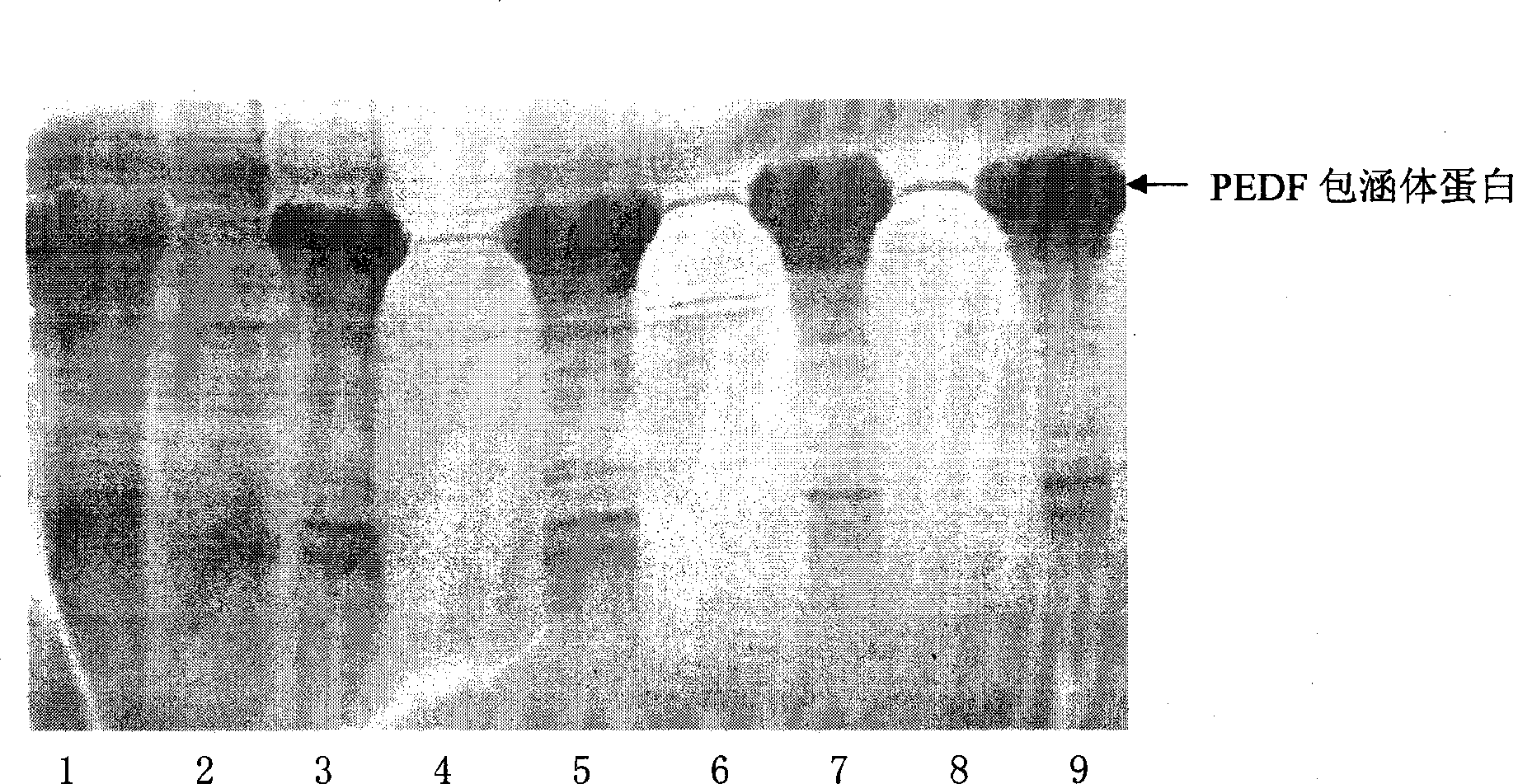
[0050] A DNA fragment encoding the polypeptide shown in SEQ NO.2 is cloned from a human cDNA library, constructed into a PET30a plasmid, transformed into a BL21(DE3) host bacterium, and expressed in the polypeptide shown in SEQ NO.2.
[0051] (2) Protein expression
[0052] Inoculate PEDF strains into 30ml LB liquid medium, culture on a shaker at 37°C and 200rpm for 5 hours, then transfer to 500ml LB liquid medium, and the culture conditions are as above. Put this culture solution into a 5L fermenter, the culture conditions are 37°C, pH 7.0, dissolved oxygen 20-30%, at OD 600 Before reaching 10, control residual sugar to 3g / L, at OD 600 After reaching 10, control the residual sugar within 1g / L. Sampling every hour to detect residual sugar and OD 600 , OD 600 Nitrogen source was added at 6 o'clock. OD 600 When it reaches about 20, add IPTG with a final co...
Embodiment 2
[0059] Example 2 N-terminal coupling of 20KD polyethylene glycol to recombinant pigment epithelium-derived factor
[0060] The recombinant human pigment epithelium-derived factor involved in this embodiment includes the recombinant human pigment epithelium-derived factor with the sequence of SEQ NO.2. The specific method is to dialyze the recombinant human pigment epithelium-derived factor into 30mM sodium acetate solution with pH 5.5, measure the protein concentration, and adjust the concentration to 3-8mg / ml. According to the molar ratio of target protein and polyethylene glycol 1 to 2, the molecular weight that needs to be added is the quality of monomethoxypolyethylene glycol propionaldehyde of 20KD, calculates the consumption of reductant CH according to final solution volume simultaneously, its concentration is 20mM. Finally, adjust the pH value of the solution to 5.1-5.3. The reaction solution was stirred evenly and left to stand at room temperature for 4-6 hours or 4...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com