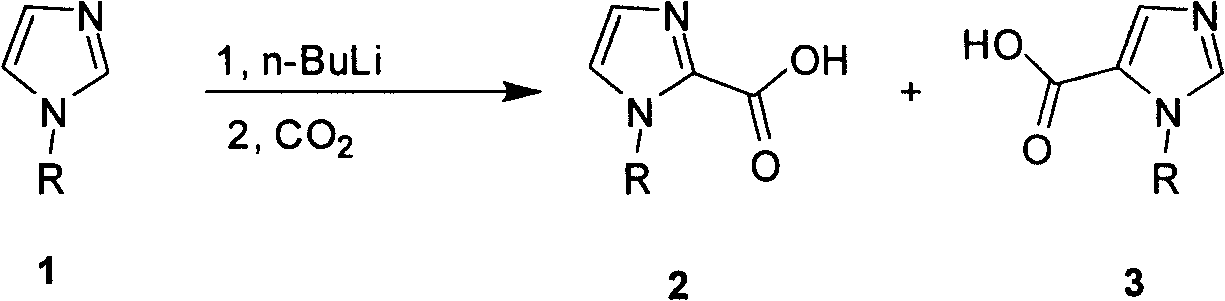
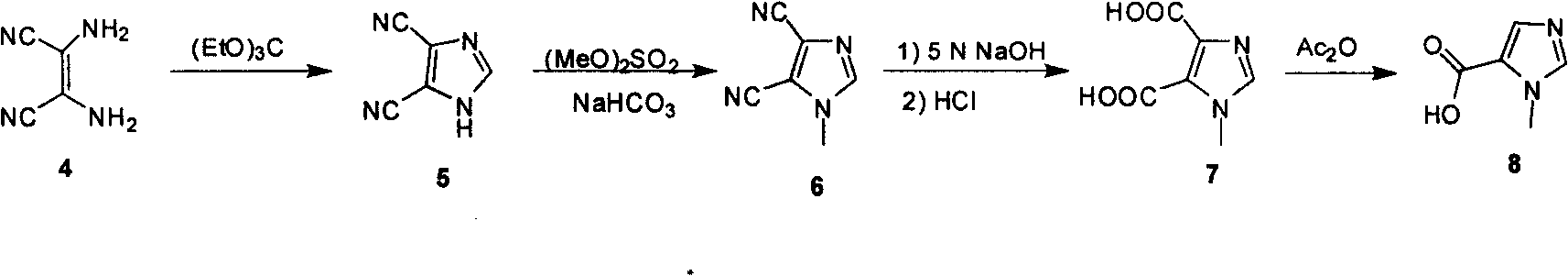
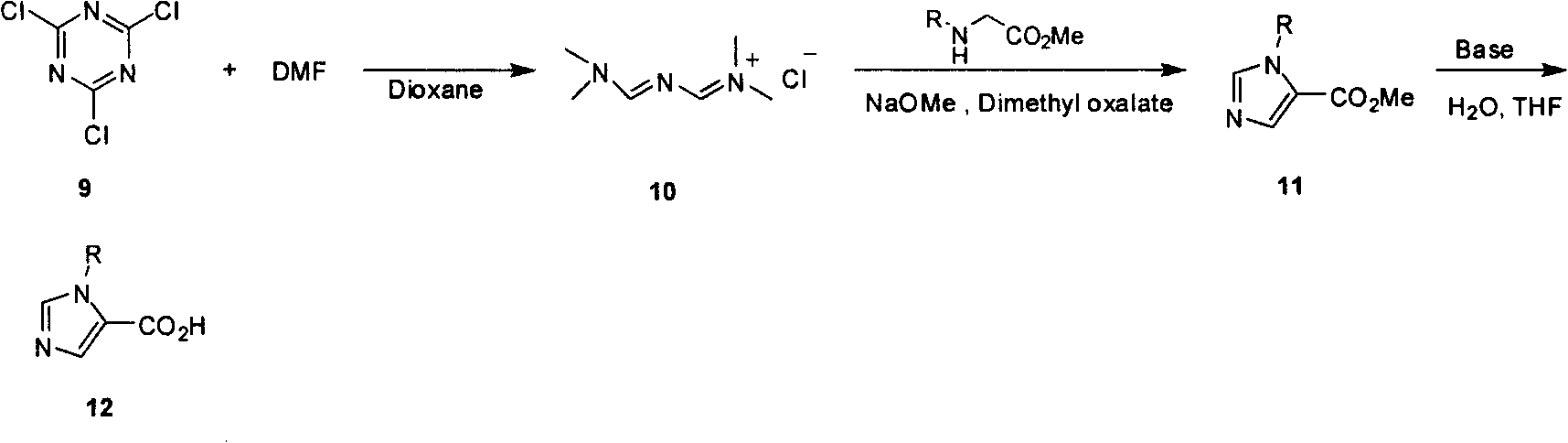
Industrialized preparation method of N-alkyl substituted-imidazole-5-carboxylic-acid/ester compound
A technology for ester compounds and alkyl groups, which is applied in the field of effectively synthesizing N-alkyl-substituted-imidazole-5-carboxylic acid/ester compounds, can solve the problems of long route, difficult purification, low yield and the like, and achieves the preparation cost. Low, reasonable selection of reaction process, easy reaction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Synthesis of N-(((dimethylamino)methenamino)methenyl)-N-dimethylammonium chloride (gold reagent)
[0032] Add 1,4-dioxane (1500mL) to cyanuric chloride (280g) and N,N-dimethylformamide (720g), heat to 65°C, react for one hour, and then heat to 75-85°C to react Two hours. Cooled to room temperature, filtered, washed the filter cake with 1,4 dioxane (1000 mL), and dried at low temperature under vacuum to obtain 650 g of product. Yield: 81%. 1 H NMR(400MHz, CDCl 3 ): δ9.58 (s, 2H, CH, CH), 3.35 (s, 6H, C 2 H 6 ), 3.17(s, 6H, CH 3 , CH 3 )
[0033] Synthesis of 1-methylimidazole-5-ethyl carboxylate
[0034] Add methyl glycine ethyl ester hydrochloride (100 g) to tert-butyl methyl ether (2500 mL), then add dimethyl oxalate (30 g) and a freshly prepared 30% sodium ethoxide ethanol solution (900 mL). Nitrogen gas was introduced into the system and gold reagent (150 g) was added at the same time to allow the reaction to occur in a nitrogen stream. Heat to 30°C to react for 24 h...
Embodiment 2
[0036] Synthesis of ethyl glycine ethyl ester hydrochloride
[0037] Ethyl ammonia (45 g) was dissolved in ethanol (400 mL), and potassium carbonate (70 g) was added. Ethyl bromoacetate (83g) was slowly added dropwise in an ice bath, and reacted at room temperature (10-15°C) for 3 hours after completion of the dropwise addition. Filter, evaporate the organic solvent, stand still and separate into layers, and separate the lower aqueous phase. Add 2N hydrogen chloride 1,4-dioxane solution (500 mL) to the upper organic matter, stir at room temperature for half an hour, filter, and wash with 1,4-dioxane (500 mL) to obtain 60 g of product, yield: 72%. 1 H NMR(400MHz, DMSO-d 6 ): δ9.48 (s, 2H, NH, HCl), 4.20 (m, 2H, CH 2 ), 3.90(m, 2H, CH 2 ), 2.93(m, 2H, CH 2 ), 1.19(m, 6H, CH 3 , CH 3 )
[0038] Synthesis of ethyl 1-ethylimidazole-5-carboxylate
[0039] Ethyl glycine ethyl hydrochloride (60 g) was added to tert-butyl methyl ether (650 mL), followed by dimethyl oxalate (10 g) and a...
Embodiment 3
[0043] Synthesis of n-pentylglycine ethyl ester hydrochloride
[0044] Glycine ethyl ester hydrochloride (14 g) was dissolved in ethanol (200 mL), cooled to 0° C., and potassium carbonate (20 g) was added. Add n-pentyl bromide (15g) dropwise, and react for 3 hours at room temperature (0-5°C) after the dropwise addition is complete. Filter, evaporate the organic solvent, add methyl tert-butyl ether solution (100 mL), stand to separate the layers, and separate the lower aqueous phase. Add 5N hydrogen chloride in dioxane solution (30 mL) to the upper organic matter, stir at room temperature for half an hour, filter, and wash with methyl tert-butyl ether (50 mL) to obtain 15 g of product. Yield: 71.4%. 1 H NMR(400MHz, DMSO-d 6 ): δ9.40 (s, 2H, NH, HCl), 4.21 (m, 2H, CH 2 ), 3.91(m, 2H, CH 2 ), 2.94(m, 2H, CH 2 ), 1.97(m, 2H, CH 2 ), 1.29-1.32 (m, 7H), 0.92 (m, 3H, CH 3 )
[0045] Synthesis of 1-n-pentylimidazole-5-ethyl carboxylate
[0046] Add n-pentylglycine ethyl ester hydrochlor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com