Endothelial cell bioreactor as well as preparation method and application thereof
A bioreactor, endothelial cell technology, applied in biochemical equipment and methods, microorganisms, biochemical instruments, etc., can solve the problems of poor growth and high requirements for endothelial cell growth environment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Example 1 Endothelial Cell Culture
[0058] Porcine iliac artery endothelial cell line (purchased from the Cell Bank of the Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences). This experiment was completed in the 3rd to 4th generation of this cell line. Pig iliac artery endothelial cells were grown in RPMI1640 medium containing 10% fetal bovine serum, double antibodies (0.0606g / L penicillin and 0.1g / L streptomycin), 2mmol / L L-glutamine, at 5% CO2, 37 ° C incubator incubation. When the PIEC cells normally cultured in the 75cm2 culture flask reached 90% confluency, they were washed once with PBS and digested by adding 0.25% trypsin-0.02% EDTA 1:1 mixture. Add 5ml of culture medium containing serum and double antibody, gently pipette to form cell suspension, and subculture.
Embodiment 2
[0059] Example 2 Preparation of non-woven endothelial cell bioreactor
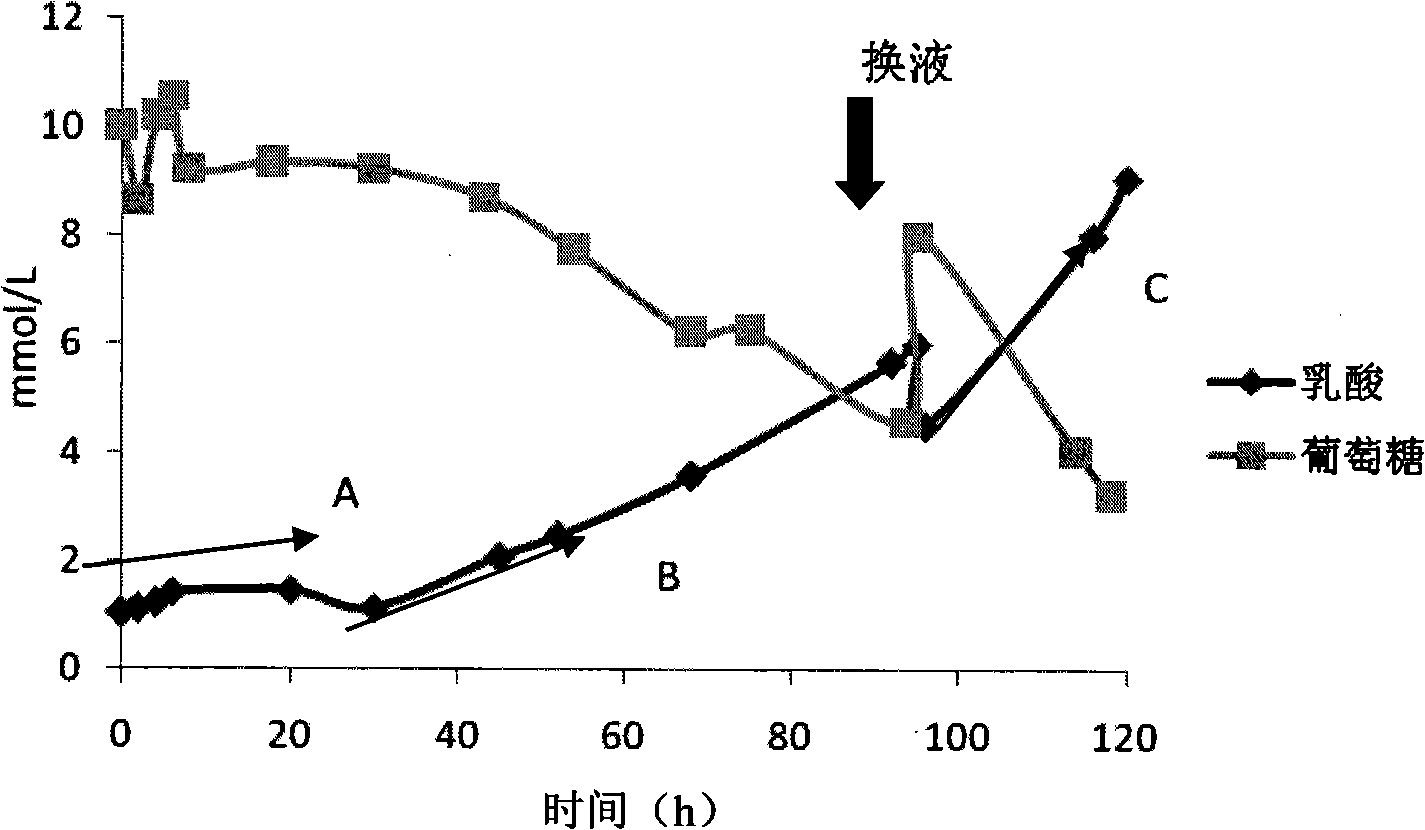
[0060] 1. Pipeline connection of the culture fluid circulation artificial capillary culture system The culture fluid circulation artificial capillary culture system is mainly composed of 4 parts: reactor, circulation pipeline, oxygenator and peristaltic pump. The specific connection method of the pipeline is as follows: Figure 5 shown. The oxygenator is equipped with 250ml RPMI 1640+10% FBS culture solution, and communicates with the outside air through a filter. Before the pipeline is connected, the inner cavity and the outer cavity of the reactor are pre-washed with physiological saline, and the outer cavity is closed after the physiological saline is emptied, and the inner cavity is connected with the circulation pipeline. After the tubing is connected, start the peristaltic pump to evacuate the gas in the inner cavity.
[0061] 2. Digestion and counting of endothelial cells when 75cm 2 After the PI...
Embodiment 3
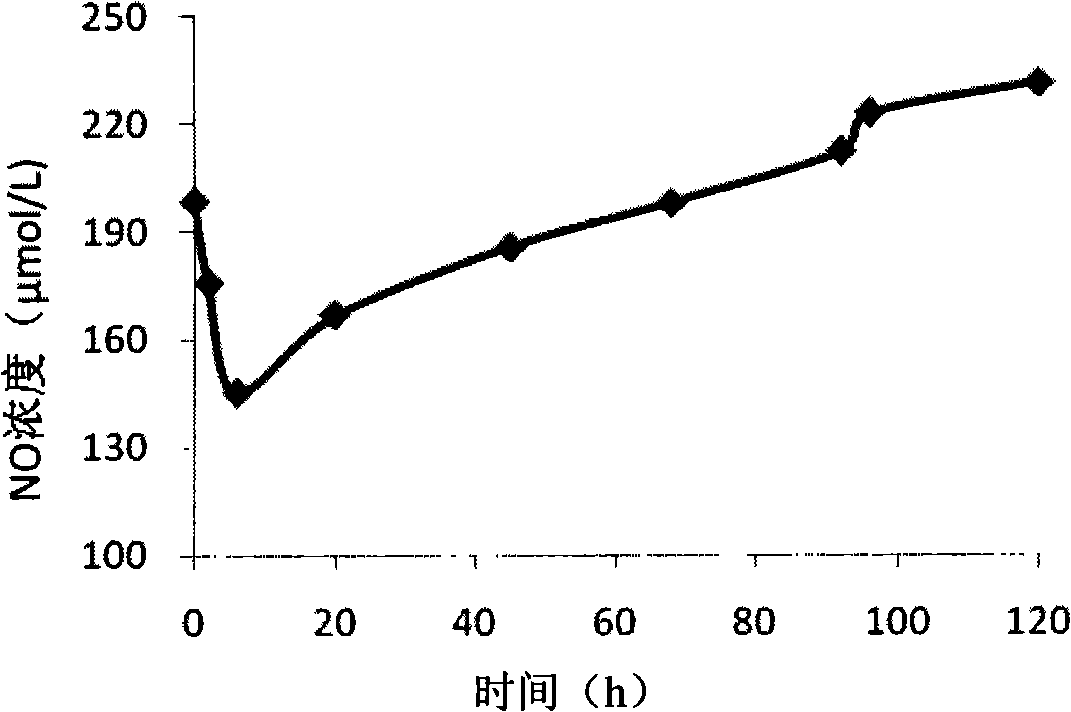
[0066] Embodiment 3 animal experiments
[0067] 1. Establishment of porcine endotoxemia model
[0068] Ten healthy hybrid domestic pigs (purchased from the Animal Experiment Center of Fudan University School of Medicine), male or female, about 3 months old, weighing 15kg-20kg, were divided into endothelial cell bioreactor group (experimental group) and pseudocirculation group ( control group) each with 5 heads. The experimental pig was anesthetized with 15 mg / kg of ketamine, and a venous cannula was placed in the ear vein, and the balance fluid was given at a rate of 20ml / kg / h, and 10ml / kg / h was maintained after 1 hour, and then the trachea was intubated and treated with isoflurane. Intravenous inhalation compound anesthesia was maintained, and then a double-lumen catheter was inserted into the internal carotid artery, and a floating catheter was placed. After stabilization, intravenous infusion of endotoxin (LPS) at a dose of 0.25 mg.kg-1 was completed within 0.5 hours, fol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com