Method for preparing epiphysin
A technology of melatonin and compounds, which is applied in the field of drug preparation, can solve the problems of restricting the industrial production of melatonin and affecting its promotion, and achieve the required conditions for the reaction and the effect of simple reaction process, low price, and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
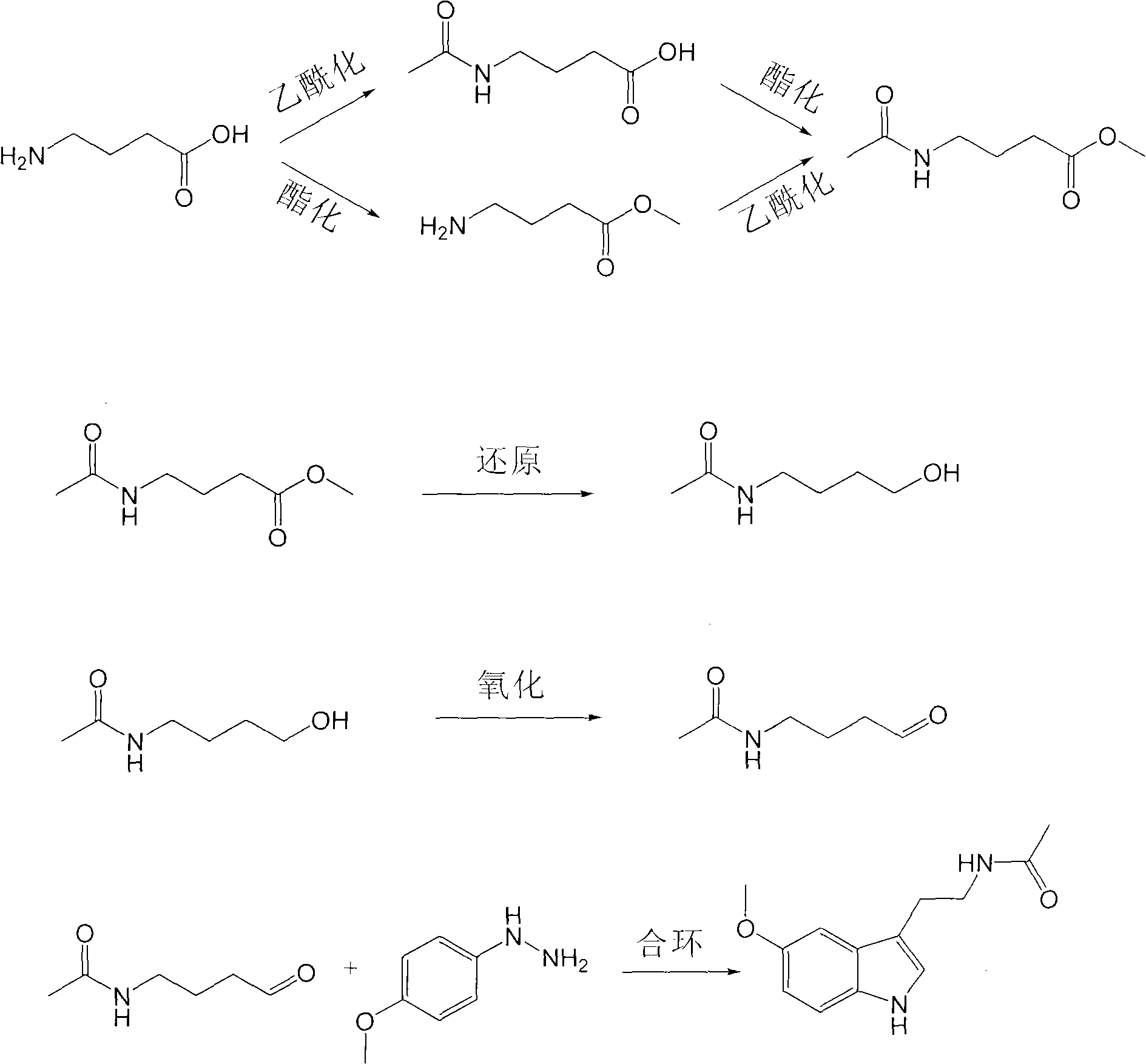
[0037] (1) Compound (II) is the synthesis of 4-aminobutyric acid methyl ester hydrochloride
[0038] Take 10.3 g of 4-aminobutyric acid, dissolve it in 150 ml of anhydrous methanol, cool it to 0-5° C. in an ice-water bath, slowly add thionyl chloride (8 ml) dropwise, and finish dropping in half an hour. Reaction at room temperature for 72h. When 20 ml of solvent remained after distillation under reduced pressure, 60 ml of anhydrous diethyl ether was added. Stir for half an hour, filter to obtain a white solid, and dry under reduced pressure to obtain 13.6 g of white crystals, with a yield of 89%. MS: m / z=118(M+1); 1 H NMR (D 2 O): 1.7-1.8 (2H, CH 2 CH 2 CH 2 ), 2.34-2.39 (2H, CH 2 CH 2 CO), 3.12-3.16 (2H, NH 2 CH 2 ), 3.64 (3H, OCH 3 ).
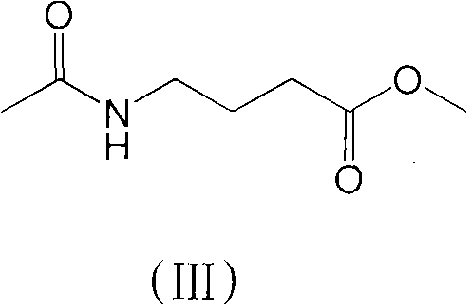
[0039] (2) Compound (III) is the synthesis of methyl N-acetylbutyrate
[0040] Take 4-aminobutyric acid methyl ester hydrochloride (15.3g), dissolve it in 500ml of dichloromethane, add 80g of anhydrous sodium carbonate, stir for ...
Embodiment 2
[0048] (1) Compound (II) is the synthesis of 4-aminobutyric acid methyl ester hydrochloride
[0049] Take 10.3 g of 4-aminobutyric acid, dissolve it in 150 ml of anhydrous methanol, cool it to 0-5° C. with an ice-water bath, and slowly add thionyl chloride (8 ml) dropwise over one hour. Reaction at room temperature for 54h. When 20 ml of solvent remained after distillation under reduced pressure, 60 ml of anhydrous diethyl ether was added. Stir for 0.5 h, filter to obtain a white solid, and dry under reduced pressure to obtain 11.5 g of white crystals, with a yield of 75%. MS: m / z=118(M+1); 1 H NMR (D 2 O): 1.7-1.8 (2H, CH 2 CH 2 CH 2 ), 2.34-2.39 (2H, CH 2 CH 2 CO), 3.12-3.16 (2H, NH 2 CH 2 ), 3.64 (3H, OCH 3 ).
[0050] (2) Compound (III) is the synthesis of methyl N-acetylbutyrate
[0051] Take 15.3g of 4-aminobutyric acid methyl ester hydrochloride, dissolve it in 500ml of dichloromethane, add 80g of anhydrous sodium carbonate, stir for 0.5h, cool to 0-5°C in ...
Embodiment 3
[0060] (1) Compound (II) is the synthesis of 4-aminobutyric acid methyl ester hydrochloride
[0061] Take 10.3g of 4-aminobutyric acid, dissolve it in 150ml of anhydrous methanol, cool it to 5-10°C with an ice-water bath, slowly add thionyl chloride (6ml) dropwise, and drop it in half an hour. Reaction at room temperature for 24h. When 20 ml of solvent remained after distillation under reduced pressure, 60 ml of anhydrous diethyl ether was added. Stir for 0.5 h, filter to obtain a white solid, and dry under reduced pressure to obtain 9.8 g of white crystals, with a yield of 62%. MS: m / z=118(M+1); 1 H NMR (D 2 O): 1.7-1.8 (2H, CH 2 CH 2 CH 2 ), 2.34-2.39 (2H, CH 2 CH 2 CO), 3.12-3.16 (2H, NH 2 CH 2 ), 3.64 (3H, OCH 3 ).
[0062] (2) Compound (III) is the synthesis of methyl N-acetylbutyrate
[0063] Take 4-aminobutyric acid methyl ester hydrochloride (15.3g), dissolve it in 500ml of dichloromethane, add 80g of anhydrous sodium carbonate, stir for 0.5h, cool to 0-5°...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com