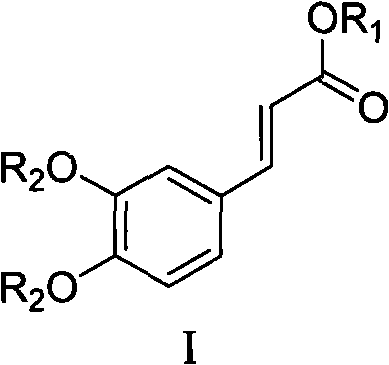
Caffeic acid diester compounds and prepartion method thereof, and application of preparing medicine for curing thrombus
A caffeic acid and compound technology, which is applied in the preparation of organic compounds, the preparation of carboxylic acid esters, chemical instruments and methods, etc., can solve the problem that acetylsalicylic acid has large adverse reactions, can not exert good efficacy, and has low bioavailability. To achieve the effect of strong antithrombotic pharmacological effect, reduced clinical dosage, and high bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
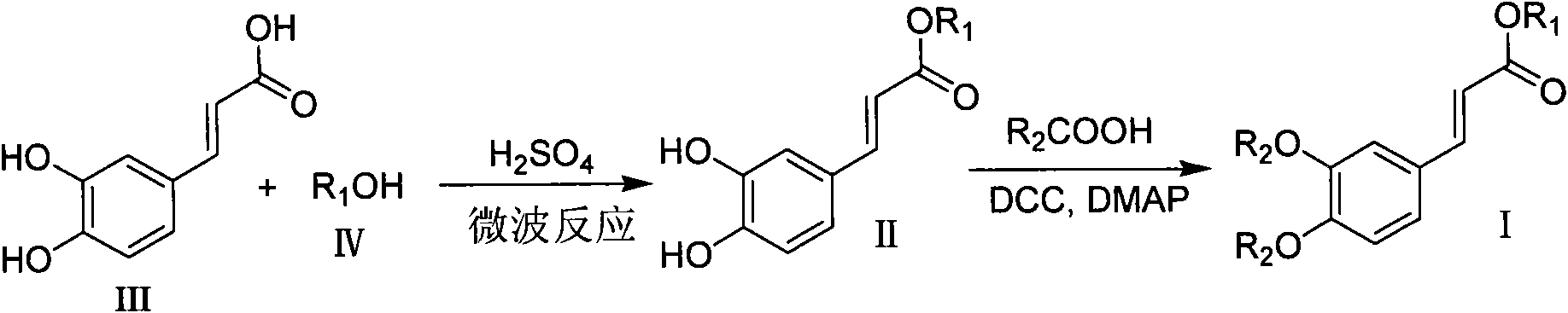
[0037] Embodiment 1 The preparation of methyl-3-(3,4-(2-acetoxy-benzoyloxy)phenyl)-acrylate
[0038] (1) Preparation of methyl caffeate
[0039] Caffeic acid (0.9 g, 5 mmol) was completely dissolved in methanol (10 ml), then concentrated sulfuric acid (0.067 ml, 1.25 mmol) was added, and the reaction was carried out in a microwave reactor with the temperature set at 100 ° C and the pressure set at 180 PSI , power set at 200W, microwave reaction for 4 minutes, cooling, adjust the pH value to neutral with saturated sodium carbonate aqueous solution, then extract with ethyl acetate, then wash with water and saturated brine successively, and finally obtain the purified product with n-hexane After recrystallization, 0.91 g of a colorless solid was obtained, with a yield of 94%. get the product 1 H NMR (300MHz, DMSO-d 6 )δ3.69(s, 3H), 6.27(d, J=15.9Hz, 1H), 6.76(d, J=8.0Hz, 1H), 7.00(dd, J 1 =2.0Hz,J 2 =8.0Hz, 1H), 7.05(d, J=2.0Hz, 1H), 7.48(d, J=15.9Hz, 1H), 9.13(s, 1H), 9.57(...
Embodiment 2
[0042] Embodiment 2 Preparation of ethyl-3-(3,4-(2-acetoxy-benzoyloxy)phenyl)-acrylate
[0043] (1) Preparation of ethyl caffeate
[0044] Caffeic acid (0.9 g, 5 mmol) was completely dissolved in absolute ethanol (10 ml), then added concentrated sulfuric acid (0.067 ml, 1.25 mmol), placed in a microwave reactor for reaction, the temperature was set at 100 ° C, the pressure was set Set at 180PSI, power at 200W, microwave reaction for 4 minutes, cool down, adjust the pH value to neutral with saturated sodium carbonate aqueous solution, then extract with ethyl acetate, then wash with water and saturated saline in sequence, and finally obtain the purified product with Recrystallized from n-hexane to obtain 0.97 g of a colorless solid with a yield of 93%. product of 1 H NMR (300MHz, DMSO-d 6 )δ1.24(t, 3H), 4.15(q, 2H), 6.25(d, J=15.9Hz, 1H), 6.76(d, J=8.2Hz, 1H), 7.01(m, 2H), 7.47( d, J=15.9Hz, 1H), 9.31(b, 2H); ESI-MS m / z: 209[M+H] + (100), indicating that the product was eth...
Embodiment 3
[0047] Example 3 Preparation of n-propyl-3-(3,4-(2-acetoxy-benzoyloxy)phenyl)-acrylate
[0048] (1) Preparation of Propyl Caffeate
[0049] Caffeic acid (0.9 g, 5 mmol) was completely dissolved in n-propanol (10 ml), then added concentrated sulfuric acid (0.067 ml, 1.25 mmol), placed in a microwave reactor for reaction, the temperature was set at 100 ° C, the pressure was set Set at 180PSI, power at 200W, microwave reaction for 5 minutes, cool down, adjust the pH value to neutral with saturated sodium carbonate aqueous solution, then extract with ethyl acetate, then wash with water and saturated saline in sequence, and finally obtain the purified product with After recrystallization from n-hexane, 1.03 g of a colorless solid was obtained, with a yield of 93%. That 1 H NMR (300MHz, DMSO-d 6 )δ0.92(t, 3H), 1.64(q, 2H), 4.07(t, 2H), 6.26(d, J=15.9Hz, 1H), 6.75(d, J=8.0Hz, 1H), 7.02( m, 2H), 7.47(d, J=15.9Hz, 1H), 9.11(s, 1H), 9.56(s, 1H); ESI-MS m / z: 223[M+H] + (100), indica...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com