Preparation method of Faropenem sodium
A technology of faropenem sodium and organic solvent is applied in the field of organic medicinal chemistry, can solve the problems of pressurization, high production cost, large amount of catalyst and the like, and achieves the effects of simple operation, low cost and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
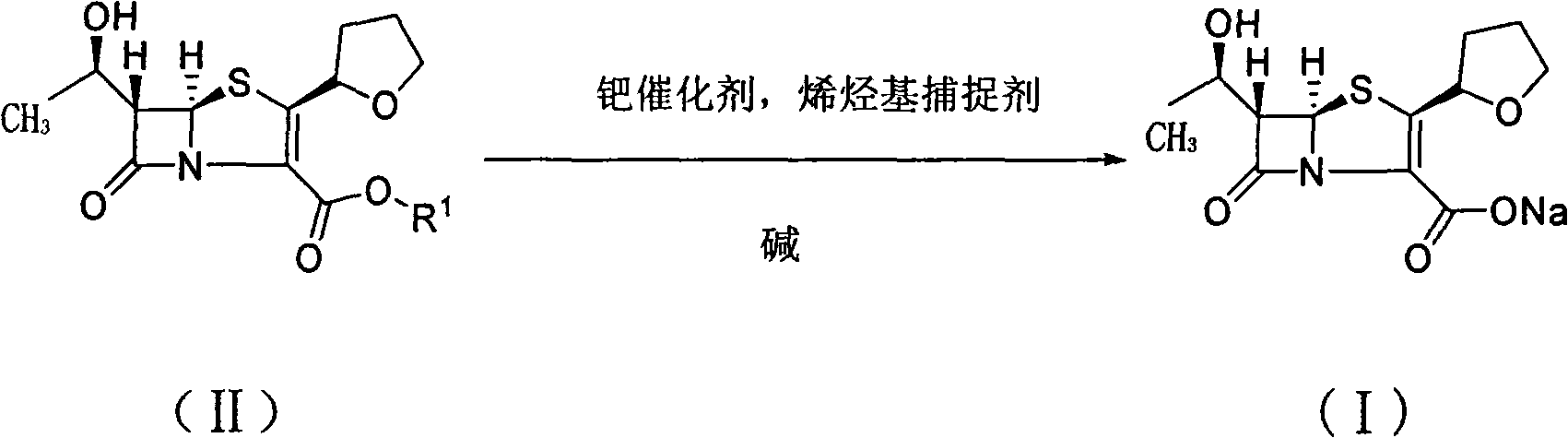
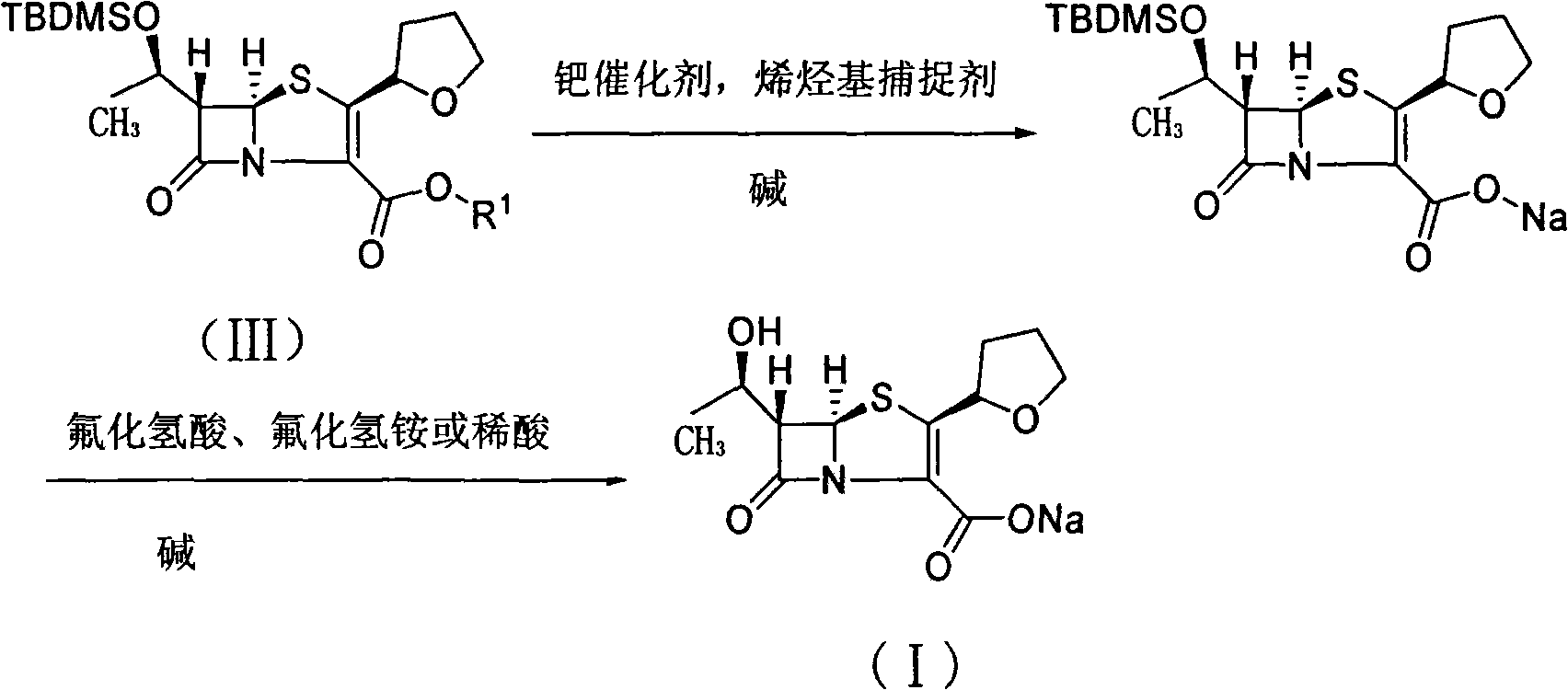
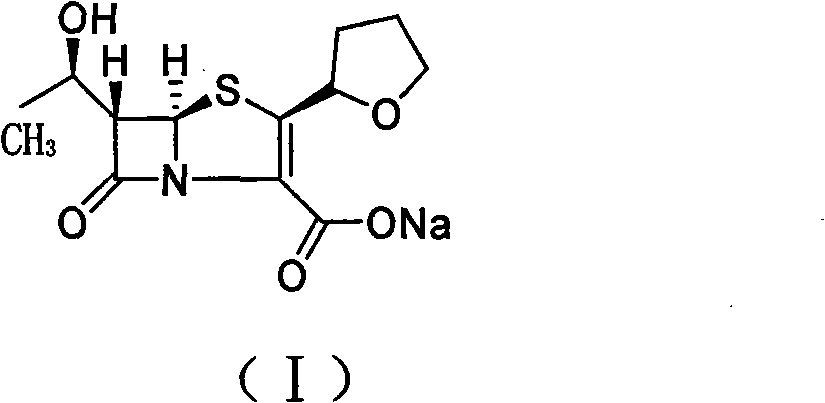
[0035] Sodium bicarbonate (5.2g, 61.4mmol) and deionized water (8mL) were placed in a reaction flask and stirred, and the olefin-based capture agent 5,5-dimethylcyclohexanedione (5.2g, 37.2mmol) was added in batches , continue to stir until the reaction solution is clear after the addition is complete; 3-Allyl carboxylate (20g, 61.4mmol) and ethyl acetate (80mL) solution, and after replacing the air in the reaction flask with nitrogen, quickly add palladium catalyst palladium dichloride (0.25g, 1.43mmol) and three Phenylphosphine (0.674g, 2.57mmol), stirred at 25°C for 5 hours, then cooled to 0°C and continued to stir for 0.5 hours, filtered, the filter cake was washed with ethyl acetate, washed with acetone-water (5:1, 240 mL) to obtain faropenem sodium (I) as a white crystalline powder. mp 163.1~164.2℃, [α] D25 =147.5°(c1.0, H 2 O).
[0036] Catalyst recovery: After the filtrate is decompressed to recover the solvent, it is washed with deionized water and dried to recove...
Embodiment 2
[0038] Sodium isooctanoate (10.2g, 61.4mmol) and deionized water (8mL) were stirred and dissolved in the reaction flask, and the olefin-based capture agent 5,5-dimethylcyclohexanedione (5.2g, 37.2 mmol), continue to stir until the reaction solution is clear after the addition; then add (1′R, 2″R, 5R, 6S)-6-[(1′-hydroxyethyl)-2″-tetrahydrofuryl] Penicillium Alkene-3-carboxylic acid allyl ester (20g, 61.4mmol) and acetone (80mL) solution, and after replacing the air in the reaction flask with nitrogen, add palladium catalyst palladium dichloride (0.25g, 1.43mmol) and triphenyl Phosphine (0.674g, 2.57mmol), stirred at 25°C for 5 hours, then cooled to 0°C and continued to stir for 0.5 hours, filtered, and the filter cake was washed with ethyl acetate, washed with acetone-water (5:1, 240mL ) recrystallization to obtain white crystalline powder faropenem sodium (I). mp 163.4~164.2℃, [α] D 25 =146.5°(c1.0, H 2 O).
[0039] Catalyst recovery: After the filtrate is decompressed to...
Embodiment 3
[0041] Sodium bicarbonate (5.2g, 61.4mmol) and deionized water (8mL) were placed in a reaction flask and stirred, and the olefin-based capture agent 5,5-dimethylcyclohexanedione (5.2g, 37.2mmol) was added in batches , continue to stir until the reaction solution is clear after the addition is complete; 3-Allyl carboxylate (20g, 61.4mmol) and ethyl acetate (80mL) solution, and after replacing the air in the reaction flask with nitrogen, quickly add the palladium catalyst bis(triphenylphosphine) palladium dichloride (1.0g , 1.43mmol), stirred at 25°C for 5 hours, then cooled to 0°C and continued to stir for 0.5 hours, filtered, washed the filter cake with ethyl acetate, and recrystallized with acetone-water (5:1, 240mL) to obtain White crystalline powder faropenem sodium (I). mp 163.3~164.2℃, [α] D 25 =147.5°(c1.0, H 2 O).
[0042] Catalyst recovery: After the filtrate is decompressed to recover the solvent, it is washed with deionized water and dried to recover the catalys...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com