Fluorescent probe for detecting glutathione reductase and bioactive sulfhydryl compound as well as synthesis method and application thereof
A technology of mercapto compounds and biological activity, which is applied in the fields of mercury organic compounds, chemical instruments and methods, biological testing, etc., and can solve the problems that chemical sensing molecules are rarely reported.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] (1) Dissolve 2.5g of 7-N, N-diethylaminocoumarin-3-aldehyde in dry methanol, slowly add 3.5g of o-mercaptoaniline dropwise under stirring and reflux reaction, filter after stirring and reflux reaction for 5 hours, and The obtained solid was vacuum-dried to obtain the probe precursor (II-1) with a yield of 87%.
[0073] EI-MS, m / e, 319.1[M+1] + .λ ab. max / nm=390nm.
[0074] (2) Dissolve 3 g of the probe precursor (II-1) in dry chloroform, and slowly add 2 g of HgCl dropwise under ice bath conditions 2 , stirred and refluxed for 3 hours to obtain a red solid. After filtration, the red solid was vacuum-dried at room temperature, and then crystallized with ethanol to obtain a red needle-shaped fluorescent probe (I-1), with a yield of 85%. EI-MS, m / e, 590.1[M+1] + .λ ab. max / nm=490nm.
[0075]
[0076] Structure of Fluorescent Probe I-1
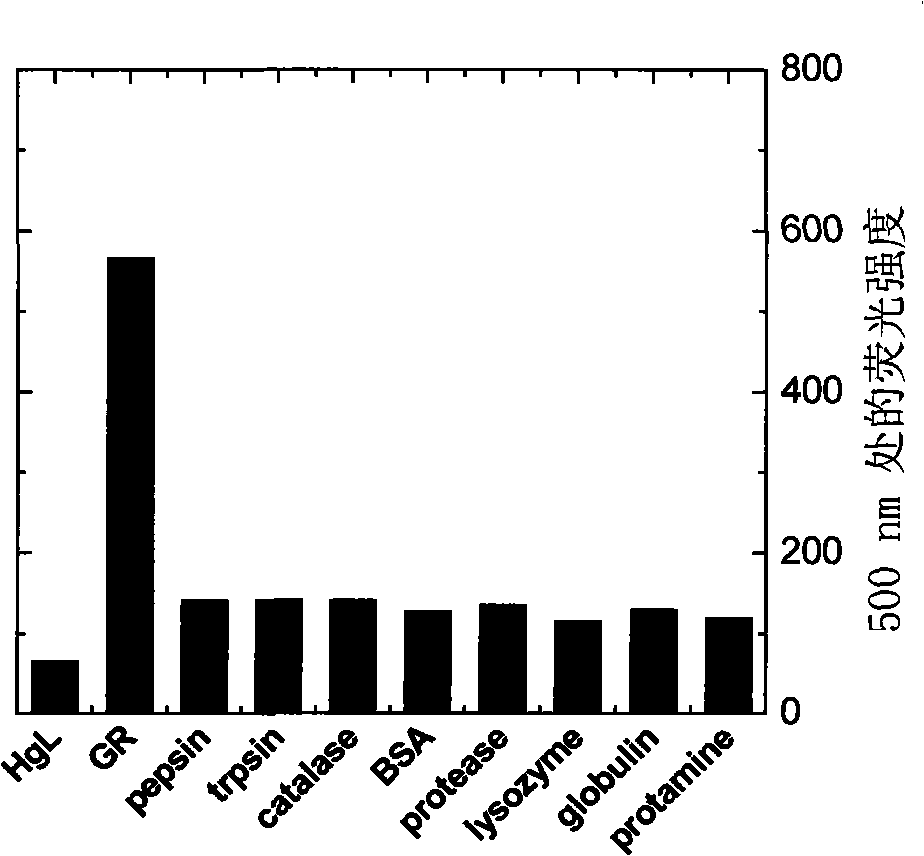
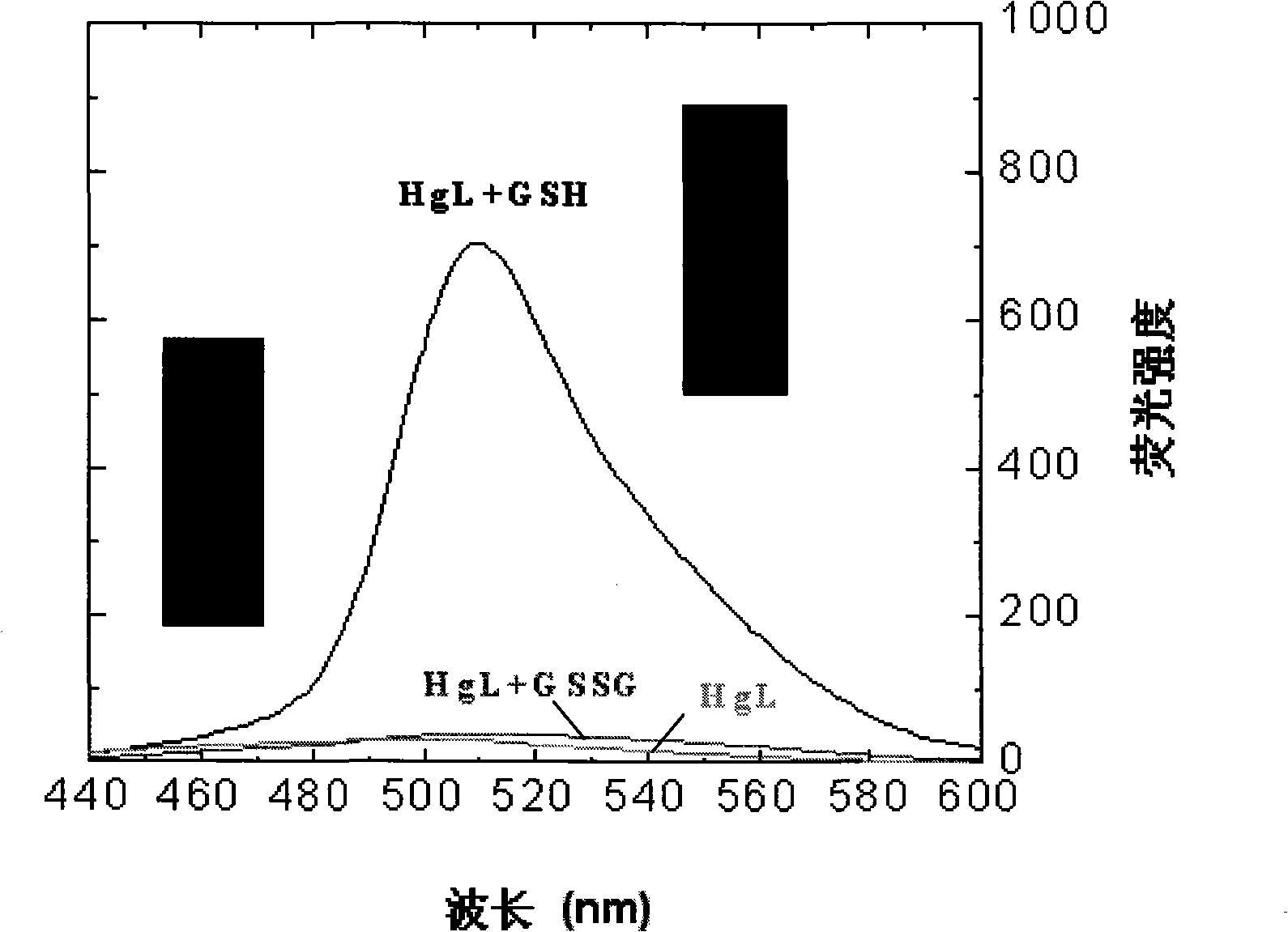
[0077] Add the fluorescent probe (I-1) to various enzymes or proteins (see Figure 1b ) solution, by Figure 1a It can be s...
Embodiment 2
[0080] (1) Dissolve 2.7g of 7-N, N-dimethylaminocoumarin-3-aldehyde in dry methanol, slowly add 5g of o-mercaptoaniline dropwise under stirring at room temperature, reflux and stir for 8 hours, then filter, and dry under vacuum at room temperature The probe precursor (II-2) was obtained after the solid, and the yield was 78%.
[0081] EI-MS, m / e, 287.1[M+1] + .λ ab. max / nm=390nm.
[0082] (2) Dissolve 3.6g of the probe precursor (II-2) in dry chloroform, and slowly add 2g of HgCl dropwise under ice bath conditions 2 After 7 hours of stirring and reflux reaction, a red solid was obtained. After filtration, the red solid was vacuum-dried at room temperature, and then crystallized with a mixture of tetrahydrofuran (THF) and ether (volume ratio 2: 1) to obtain a red needle-shaped fluorescent probe (I- 2), the yield is 90%.
[0083] EI-MS, m / e, 558.1[M] + .λ ab. max / nm=487nm.
[0084]
[0085] Structure of fluorescent probe I-2
Embodiment 3
[0087] (1) Dissolve 25g of 7-N, N-dimethylamino-4-methylcoumarin-3-aldehyde in dry methanol, slowly add 32g of o-mercaptoaniline dropwise under stirring at room temperature, and filter after stirring for 4 hours. The obtained solid was vacuum dried to obtain the probe precursor (II-3) with a yield of 61%.
[0088] EI-MS, m / e, 302.2[M+1] + .λ ab. max / nm=390nm.
[0089] (2) Dissolve 13g of the probe precursor (II-3) in dry chloroform, and slowly add 10g of HgCl dropwise under ice bath conditions 2 , a red solid was obtained after stirring and reacting for 7 hours. After filtration, the red solid was vacuum-dried at room temperature, and then crystallized with a mixture of THF:ether (volume ratio 2:1) to obtain a red needle-like crystal fluorescent probe (I-3), producing The rate is 87%.
[0090] EI-MS, m / e, 573.1[M+1] + .λ ab. max / nm=481nm.
[0091]
[0092] Structure of fluorescent probe (I-3)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com