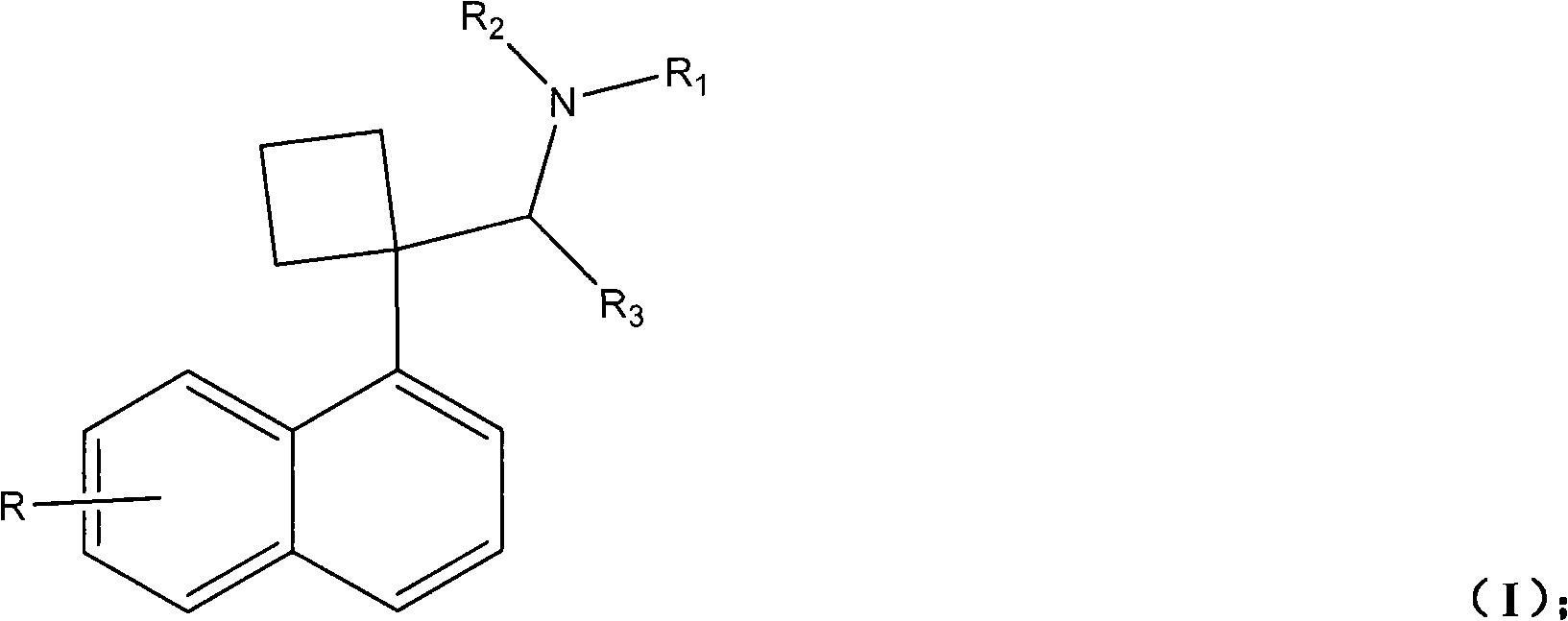
Naphthyl ethylamine derivative and preparation method thereof and application of naphthyl ethylamine derivative in preparing weight-reducing medicament
The technology of a derivative, naphthylethylamine, which is applied in the field of obesity medicine and pharmacy, can solve the problems of limited choice and insignificant curative effect, and achieve a good effect of weight loss and lipid reduction.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048]The preparation of embodiment 1 pharmaceutical intermediate of the present invention
[0049] Synthesis of (7-methoxy-3,4-dihydro-1-naphthyl)acetonitrile:
[0050] 7-methoxytetralin-1-one (17.6g, 0.1mol), cyanoacetic acid (12.8g, 0.15mol), benzylamine (2.7g, 25mmol), heptanoic acid (3.3g, 25mmol) were dropped into toluene ( 160ml), refluxed and separated water for 24 hours, cooled to room temperature, the reaction solution was washed successively with 2mol / L sodium hydroxide solution (60ml), water (60ml) and saturated brine (60ml), dried with anhydrous sodium sulfate, filtered , the filtrate was distilled off toluene to obtain an oil (18.9 g, 95%).
[0051] Synthesis of (7-methoxyl-1-naphthyl)acetonitrile:
[0052] DDQ (25g, 0.11mol) was dropped into dry dichloromethane (200ml), and a dichloromethane solution (100ml) of the above-mentioned oil (20g, 0.1mol) was added dropwise at 20°C. Filter, the filtrate was washed successively with saturated sodium bicarbonate solut...
Embodiment 2
[0056] Embodiment 2 The preparation of [1-(7-methoxy-1-naphthyl) cyclobutyl] methylamine of the present invention
[0057] Add 1-(7-methoxynaphthyl)cyclobutyronitrile (9.5g, 0.04mol), nickel chloride hexahydrate (9.5g, 0.04mol), and 30ml of absolute ethanol into a 100ml eggplant-shaped bottle under an ice bath , sodium borohydride (4.6 g, 0.12 mol) was added in batches under vigorous stirring, the color of the solution turned black, and stirring was continued for 30 minutes after addition. Then slowly add 30ml of hydrochloric acid with a concentration of 4mol / L to the reaction solution, and the color of the solution gradually changes to light green. The solution was concentrated to remove ethanol, and then filtered to remove unreacted 1-(7-methoxynaphthyl)cyclobutyronitrile. The filtrate was adjusted to pH 9-10 with sodium hydroxide, the solution was extracted with ethyl acetate (20ml×3), the ethyl acetate was combined, washed with water (30ml) and saturated brine (30ml) resp...
Embodiment 3
[0059] Embodiment 3 Preparation of 1-[1-(7-methoxy-1-naphthyl)cyclobutyl]-N,N-dimethylmethylamine of the present invention
[0060] Get [1-(7-methoxy-1-naphthyl)cyclobutyl]methylamine (2.4g, 0.01mol) in a 50ml eggplant-shaped bottle, slowly mix 88% formic acid (15ml), 37% formaldehyde Solution (5ml) was added therein, refluxed for one hour, then 37% formaldehyde (5ml) was added dropwise, continued to reflux for 24h, cooled to room temperature, poured into 40ml ice water, adjusted pH to about 9-10 with 50% sodium hydroxide, Extracted three times with ethyl acetate (20ml×3), combined ethyl acetate was washed with water (30ml) and saturated brine (30ml) respectively. Dry over anhydrous sodium sulfate, filter, and evaporate the solvent from the filtrate to obtain an orange-red oil (2.15 g, 79.6%), which becomes a salt.
[0061] 1 H-NMR (δ, ppm, d-DMSO): 2.01(m, 2H) 2.26(s, 6H); 2.52~2.79(m, 4H) 2.63(s, 2H); 3.76(s, 3H); 7.10~ 7.75 (m, 6H)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com