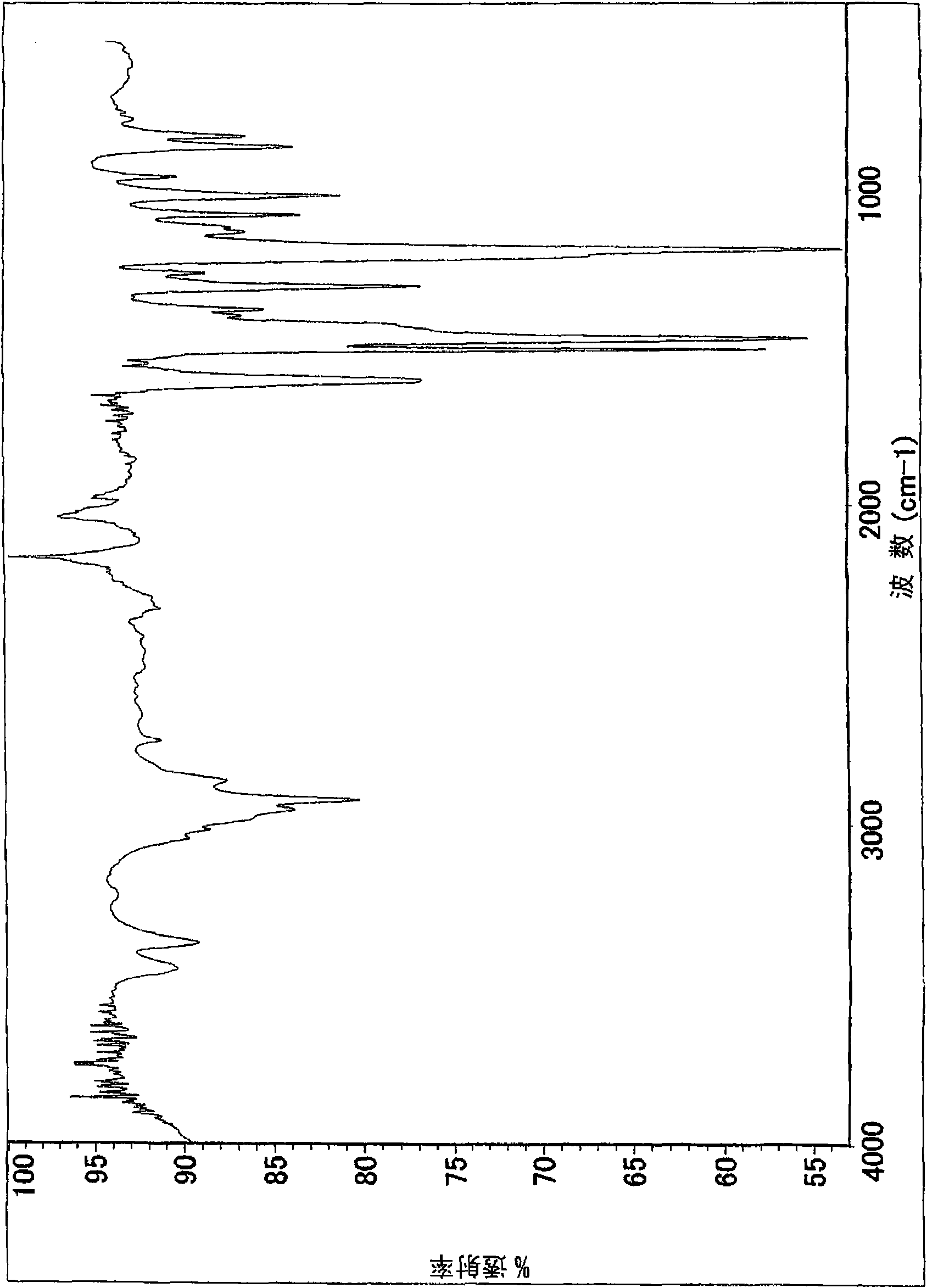
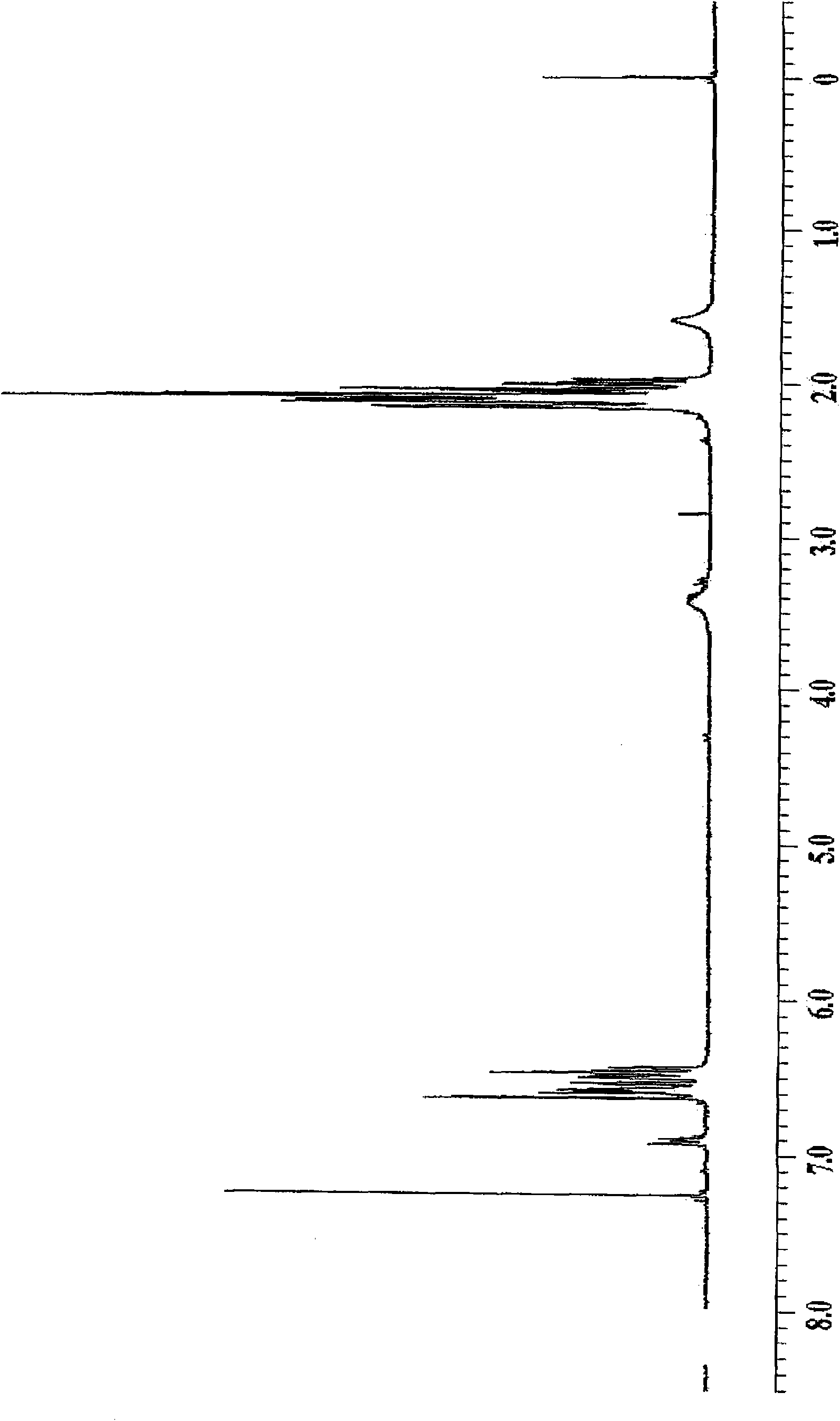
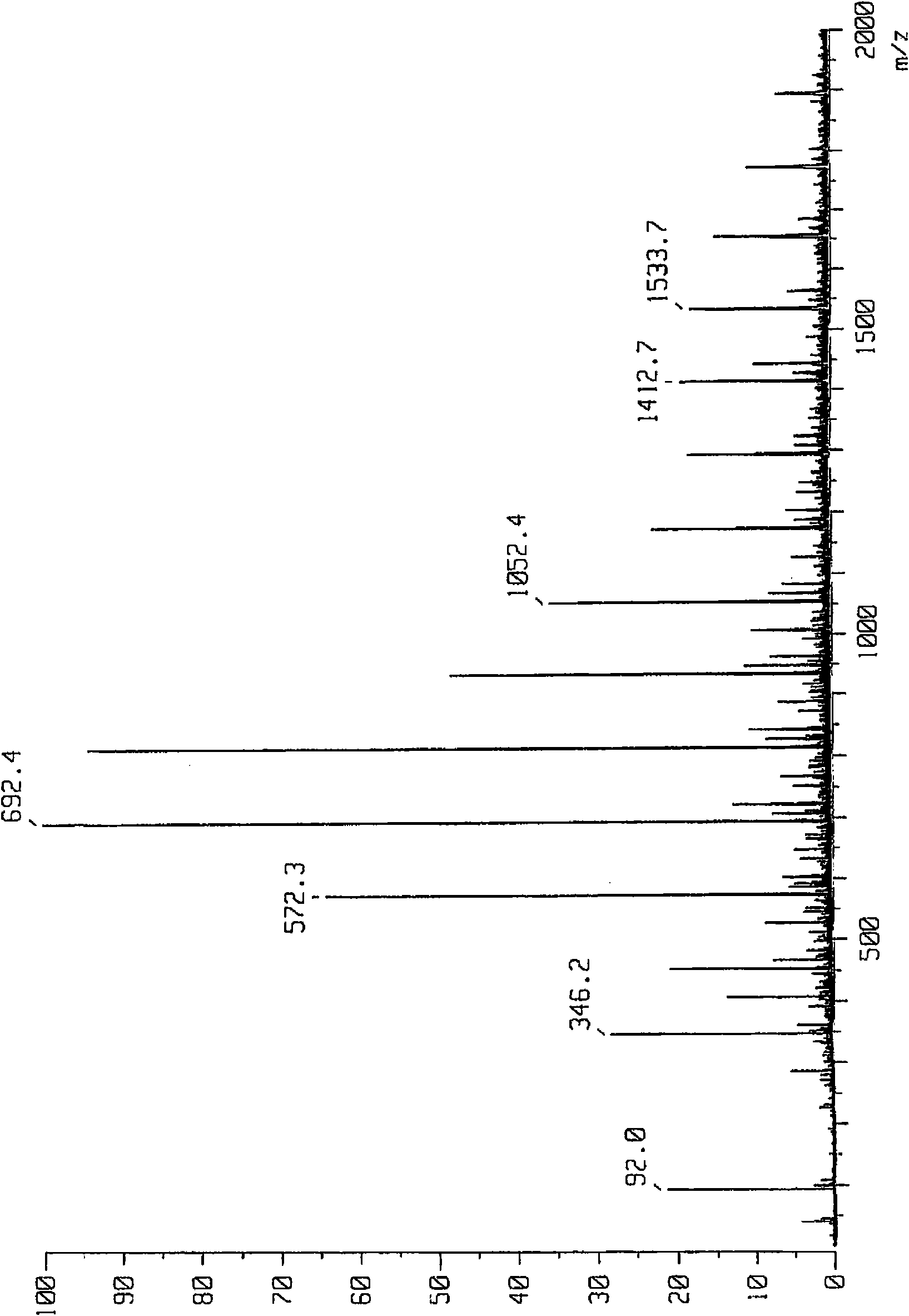
Bismaleamic acid, bismaleimide and cured product thereof
A technology of bismaleimide and bismaleamic acid, which is applied in the field of bismaleamic acid, bismaleimide and their cured products, can solve the problems of difficult high performance requirements and the like, and achieves the Less variation in dielectric properties, low water absorption, and effects of low dielectric properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0091] The present invention will be described more specifically by way of examples below, but the present invention is not particularly limited to the following examples. In addition, the number average molecular weight and weight average molecular weight were calculated|required by the gel permeation chromatography (GPC) method (polystyrene conversion). As a developing solvent for GPC, THF (tetrahydrofuran) was used. The hydroxyl equivalent and amino equivalent were obtained by quantifying the terminal functional groups by titration. The dielectric constant was measured at 10 GHz by the cavity perturbation method. In the examples of the present invention, drying refers to a state in which calcium chloride is placed in a desiccator (humidity 21%) at a temperature of 20°C for 12 hours, and moisture absorption refers to immersion in water at a temperature of 25°C for 24 hours. hour status. The glass transition temperature is obtained by the DMA method for the cured product, ...
Synthetic example 1
[0093] Synthesis of 2-functional phenylene ether oligomers
[0094] In a 12L vertical elongated reactor with a stirring device, a thermometer, an air inlet pipe, and a baffle, drop 3.88g (17.4mmol) of CuBr 2 , 0.75g (4.4mmol) N, N'-di-tert-butylethylenediamine, 28.04g (277.6mmol) n-butyldimethylamine, 2600g toluene, stirring at a reaction temperature of 40°C, will be mixed by nitrogen and air Mix and adjust the mixed gas with an oxygen concentration of 8% to bubble at a flow rate of 5.2 L / min, and add dropwise 129.32 g (0.48 mol) of 2,2', 3,3 previously dissolved in 2300 g of methanol for 230 minutes. ', 5,5'-hexamethyl-(1,1'-biphenyl)-4,4'-diol, 292.19g (2.40mol) 2,6-dimethylphenol, 0.51g (2.9mmol ) N, N'-di-tert-butylethylenediamine, 10.90g (108.0mmol) mixed solution of n-butyldimethylamine, and stirred. After completion of the dropwise addition, 1500 g of water in which 19.89 g (52.3 mmol) of tetrasodium ethylenediaminetetraacetate was dissolved was added to terminate t...
Synthetic example 2
[0100] Synthesis of 2-functional phenylene ether oligomers
[0101] In a 12L vertical elongated reactor with a stirring device, a thermometer, an air inlet pipe, and a baffle, drop 9.36g (42.1mmol) of CuBr 2, 1.81g (10.5mmol) N, N'-di-tert-butylethylenediamine, 67.77g (671.0mmol) n-butyldimethylamine, 2600g toluene, stirring at a reaction temperature of 40°C, will be mixed by nitrogen and air Mix and adjust the mixed gas with an oxygen concentration of 8% to bubble at a flow rate of 5.2 L / min, and add dropwise 129.32 g (0.48 mol) of 2,2', 3,3 previously dissolved in 2300 g of methanol for 230 minutes. ', 5,5'-hexamethyl-(1,1'-biphenyl)-4,4'diol, 878.4g (7.2mol) 2,6-dimethylphenol, 1.22g (7.2mmol) A mixed solution of N,N'-di-tert-butylethylenediamine and 26.35 g (260.9 mmol) of n-butyldimethylamine was stirred. After completion of the dropwise addition, 1500 g of water in which 48.06 g (126.4 mmol) of tetrasodium ethylenediaminetetraacetate was dissolved was added to termin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com