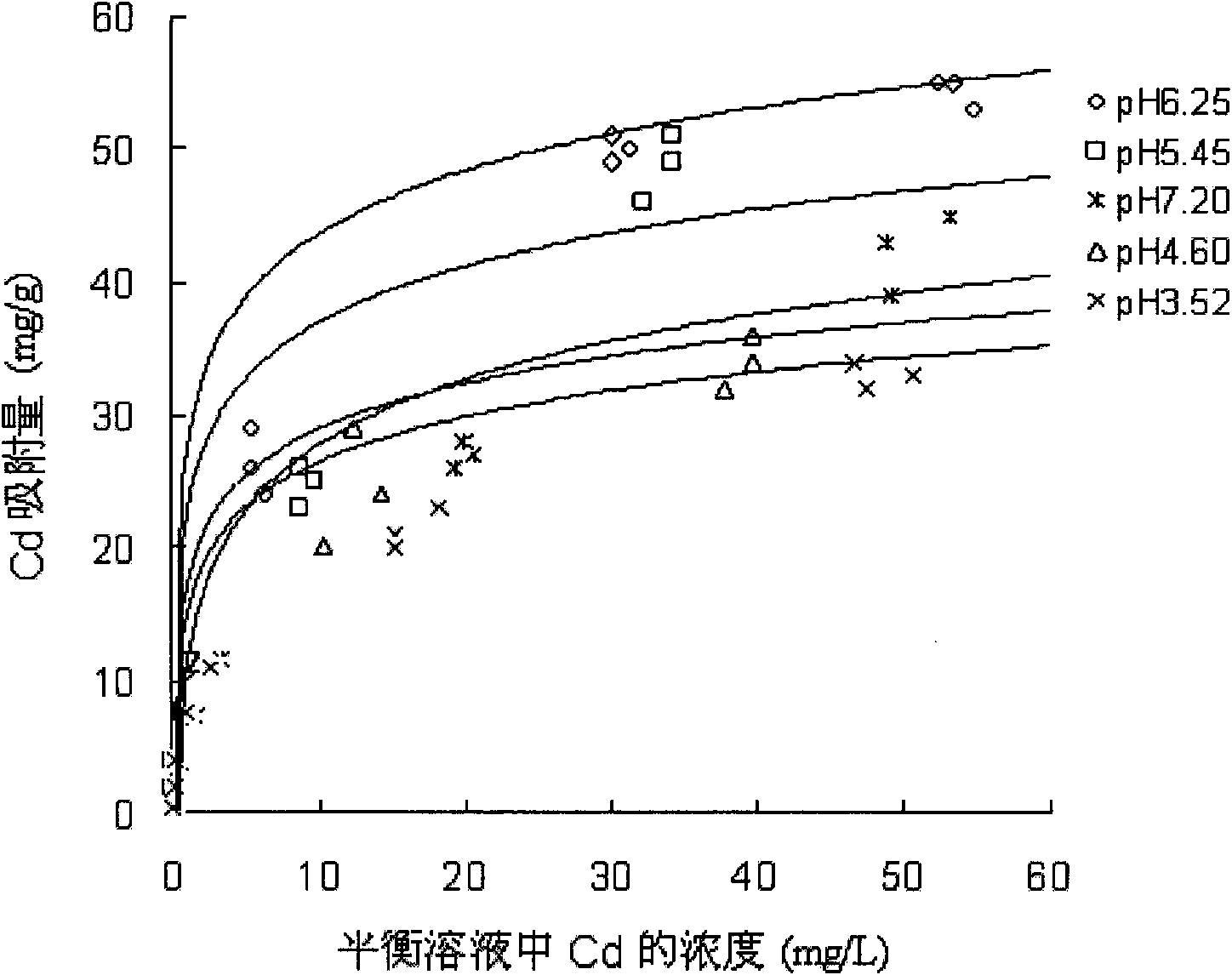
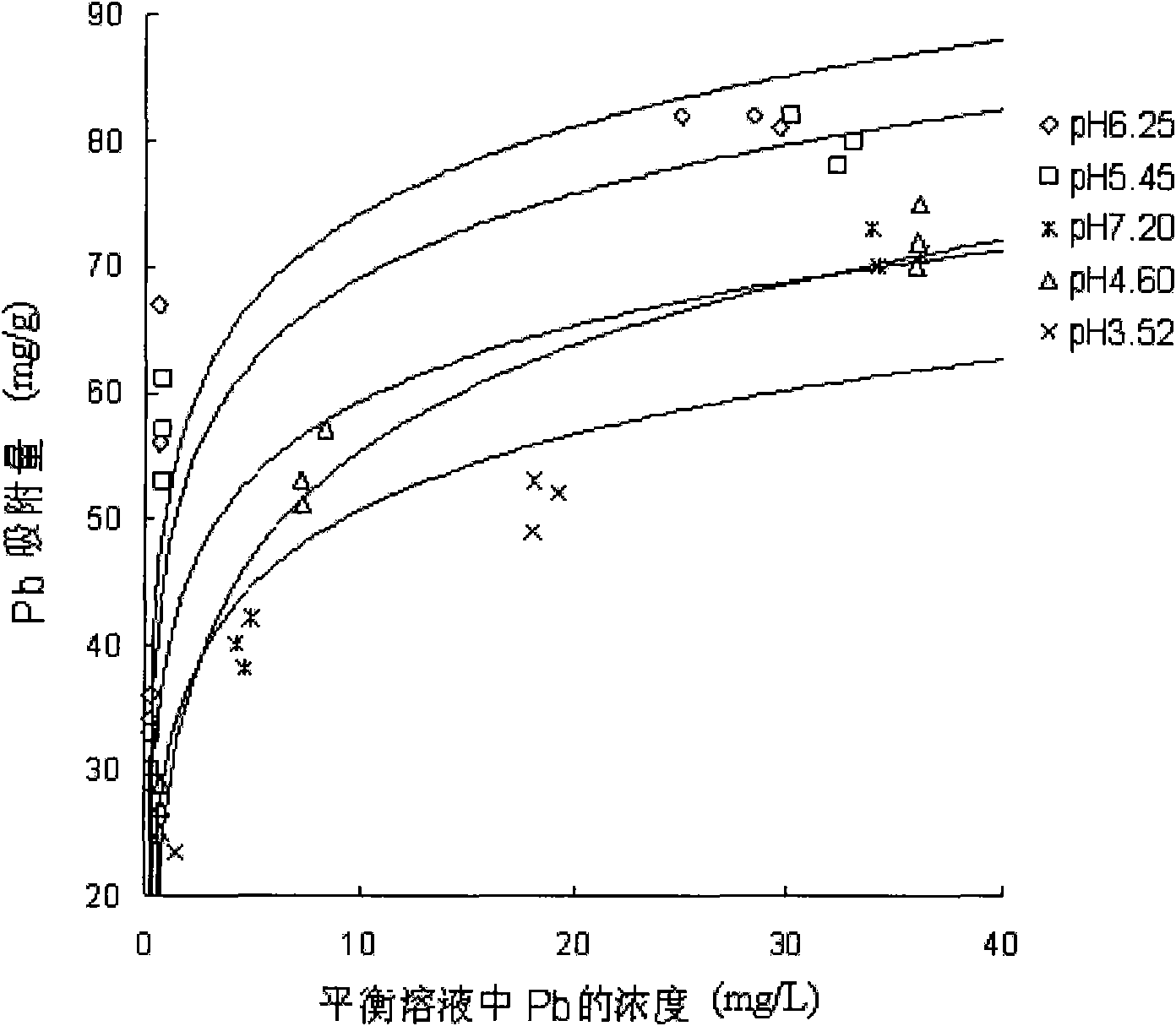
Method for removing heavy metal ions in sewage by nanometer hydroxylapatite
A nano-hydroxyapatite, heavy metal ion technology, applied in water/sewage treatment, chemical instruments and methods, adsorption water/sewage treatment, etc., can solve the problem of heavy metal removal efficiency that has not been reported, and achieve strong on-site operability , the effect of small environmental risk and high governance efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Using the above mother liquor, sequentially prepare Cd(NO 3 ) 2 solution, Pb(NO 3 ) 2 solution and Cu(NO 3 ) 2 solution, and then take 30mL of each solution and add them to the 50mL plastic centrifuge tube containing 0.20g of the above-mentioned nano-hydroxyphosphate rock powder, wherein, 0.05mol / L KNO 3 As a supporting electrolyte, 0.05M HNO 3 / KOH to adjust the pH of the solution. Each treatment was repeated 3 times. Cover and seal the centrifuge tube with the sample added, shake at a constant temperature at 25°C for 24 hours, then stand still and centrifuge (4000r·min -1 ) for 15 minutes, filter, and measure the concentration of Cd in the solution after equilibrium with atomic absorption spectrophotometry (AAS / FAAS) method, Pb, Cu ions. According to the concentration difference of Cd, Pb and Cu ions in the initial solution and the equilibrium solution, calculate the removal rate R of hydroxyphosphate powder for Cd, Pb and Cu ions (see Equation 1):
[0024] ...
Embodiment 2
[0038] Preparation of Cd(NO 3 ) 2 , Pb(NO 3 ) 2 and Cu(NO 3 )2 The mixed aqueous solution, wherein Cd, Pb, Cu ion concentration is 40mg / L, pH is 6, gets this mixed solution 30mL and joins respectively in the 50mL plastic centrifuge tube that contains 0.18g and 0.24g above-mentioned nano-hydroxyphosphate rock powder, wherein , with 0.05mol / L KNO 3 As a supporting electrolyte, 0.05M HNO 3 / KOH to adjust the pH of the solution. Each treatment was repeated 3 times. Cover and seal the centrifuge tube with the sample added, shake at a constant temperature at 27°C for 16 hours, rest, centrifuge (4000r / min) for 15 minutes, filter, and measure the balance by atomic absorption spectrophotometry (AAS / FAAS) The concentration of Cd, Pb, Cu ions in the solution. The calculation of removal rate is with embodiment 1. The results show that when the amount of nano-hydroxyphosphate rock powder is 6g / L, the removal rate of heavy metal ions Pb in the above mixed solution is 85.4%, the rem...
Embodiment 3
[0040] Cd(NO 3 ) 2 Aqueous solution, pH is 6.5, take 30mL of this solution and join in the 50mL plastic centrifuge tube containing 0.21g above-mentioned nano-hydroxyphosphate rock powder, wherein, with 0.05mol / LKNO 3 As a supporting electrolyte, 0.05M HNO 3 / KOH to adjust the pH of the solution. Each treatment was repeated 3 times. Cover and seal the centrifuge tube with the sample added, shake at a constant temperature at 23°C for 48 hours, then rest, centrifuge (4000r / min) for 15 minutes, filter, and measure the balance by atomic absorption spectrophotometry (AAS / FAAS) The concentration of Cd ions in the solution. The calculation of removal rate is with embodiment 1. The removal rate reaches 92%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| clearance rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com