Monohydroxy conjugated linoleic acid, preparation method and application thereof
A technology of monohydroxy conjugated linoleic acid and linoleic acid, which is applied in the direction of carboxylate preparation, organic compound preparation, skin care preparations, etc., can solve the problem of ineffective effect and achieve good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0124] Into a 50 mL round bottom flask was added 383 mg of SeO 2 , 10mL of dichloromethane, stirred at 25°C for 1 hour, slowly added 1g of linoleic acid with a purity of 98%, and continued to stir for 48 hours. After the reaction, add water to wash, collect the dichloromethane layer, and recover the dichloromethane to obtain 0.90 g of light yellow oil with a yield of 70.2% and a purity of 92% (W / W).
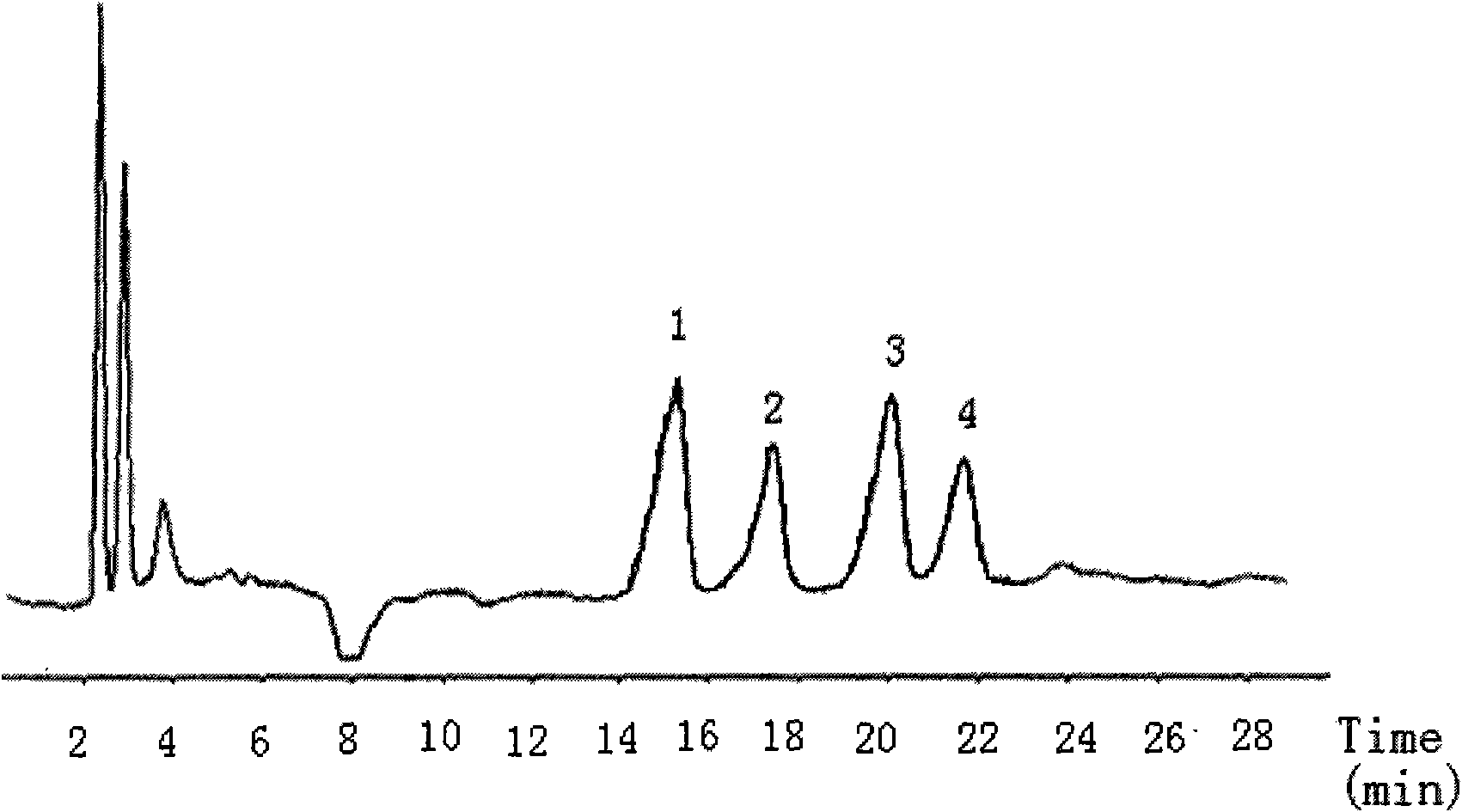
[0125] Analysis of the product: the light yellow oil obtained by the reaction is separated by semi-preparative normal phase high-performance liquid chromatography, and the separation conditions: the mobile phase is n-hexane: Virahol (volume ratio) is 99.4: 0.6; flow rate 8mL / min ; The detector is a differential refractive index detector; the sample is dissolved in n-hexane with a concentration of 100 mg / mL; the sample injection is 150 μL. Under this separation condition, four product peaks ( figure 1 ), the retention times are 15.4min (peak 1), 17.4min (peak 2), 20.1min (peak 3...
Embodiment 2
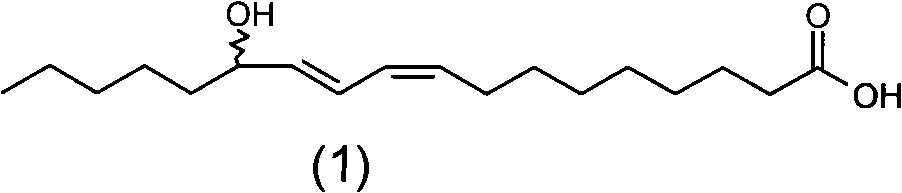
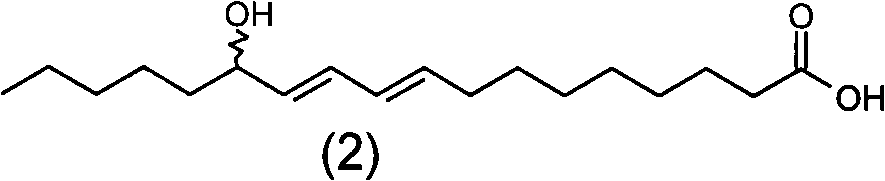
[0158] Add 200 mg of SeO to a 50 mL round bottom flask 2 , 5mL of dichloromethane, stirred at 60°C for 1 hour, slowly added 2g of linoleic acid with a purity of 90%, and continued to stir for 12 hours. After the reaction, add water to wash, collect the dichloromethane layer, and recycle the dichloromethane to obtain 0.8 g of light yellow oil with a yield of 36.4% and a purity of 88% (W / W). The product is analyzed by normal phase high performance liquid chromatography, analysis conditions: mobile phase is n-hexane: Virahol (volume ratio) is 99.4: 0.6; Flow velocity 2mL / min; Detector is differential refraction detector; Sample concentration is 20mg / min. mL; inject 10 μL. Under this separation condition, four product peaks are obtained, and the retention times are respectively 15.4min (peak 1), 17.4min (peak 2), 20.1min (peak 3), and 21.8min (peak 4). figure 1 , confirmed as compounds: 13-Hydroxy-9Z, 11E-octadecadienoic acid; 13-Hydroxy-9E, 11E-octadecadienoic acid; 9-Hydroxy-1...
Embodiment 3
[0160] Add 700mg of SeO to a 50mL round bottom flask 2 , 30mL of n-hexane, stirred at 40°C for 2 hours, slowly added 1g of linoleic acid with a purity of 75%, and continued to stir for 24 hours. After the reaction was finished, add water to wash, collect the n-hexane layer, and recover the n-hexane to obtain 0.9 g of light yellow oil with a yield of 52.9% and a purity of 70% (W / W). Product adopts normal phase high performance liquid chromatography to analyze, and analysis condition is with embodiment 2, obtains four product peaks, retention time is respectively 15.4min (peak 1), 17.4min (peak 2), 20.1min (peak 3), 21.8 min (peak 4), compared with the standard of the four isomers, confirmed to be the compound: 13-hydroxy-9Z, 11E-octadecadienoic acid; 13-hydroxy-9E, 11E-octadecadienoic acid ; 9-Hydroxy-10E, 12Z-octadecadienoic acid, 9-Hydroxy-10E, 12E-octadecadienoic acid.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com