Dispersion solution of metal nanoparticle, method for production thereof, and method for synthesis of metal nanoparticle
A technology of metal nanoparticles and synthesis methods, which can be used in cable/conductor manufacturing, electrical components, circuits, etc., and can solve problems such as raw material limitations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~20、 comparative example 1~3
[0398] First, metal salts containing metal elements shown in Table 1 and Table 2 below, carboxylic acids, reducing agents and additives were respectively dissolved in deionized water to prepare saturated aqueous solutions at room temperature. Note that nitrates were used as metal salts, and only chlorine compounds were used for Au and Pt.
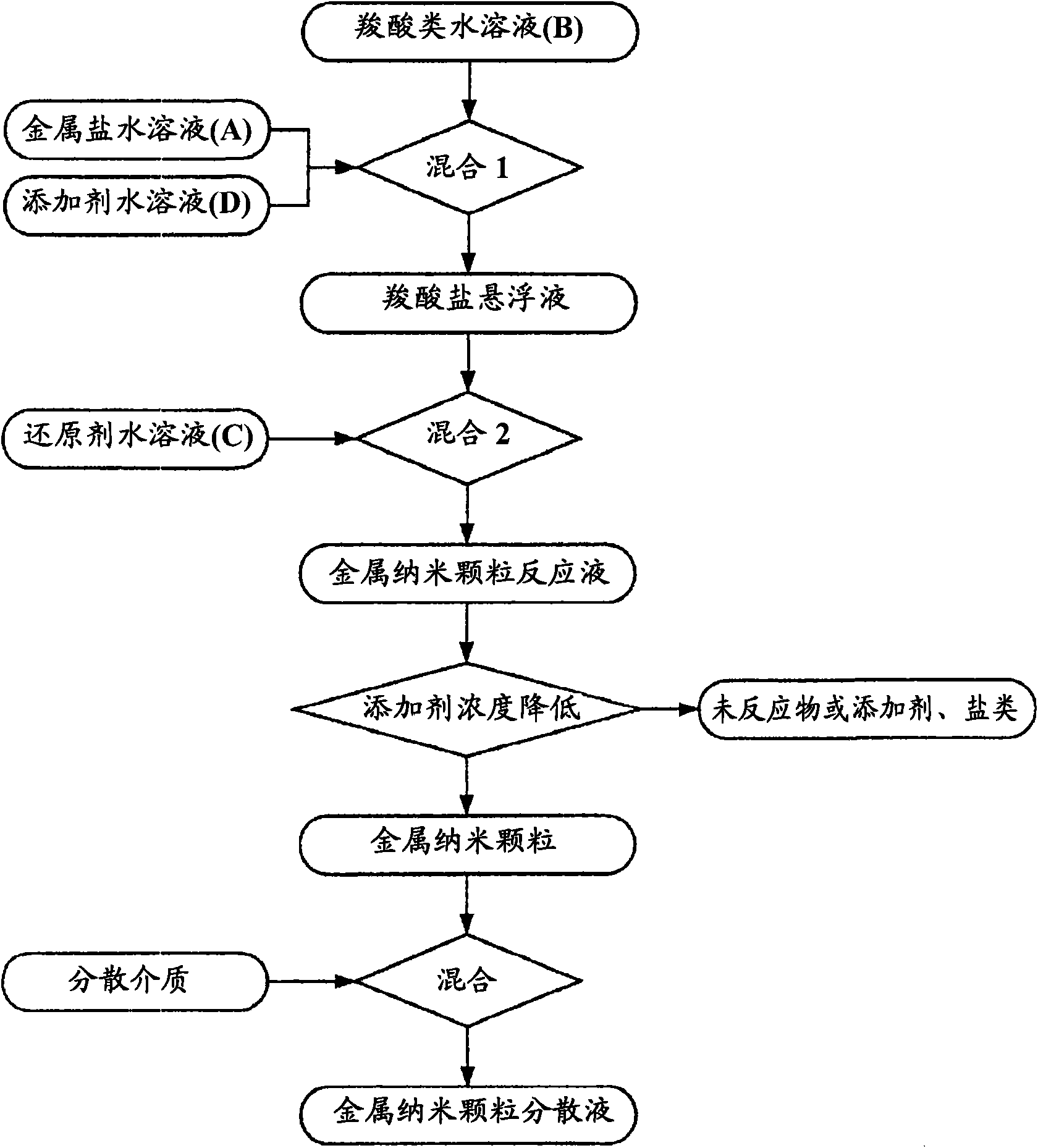
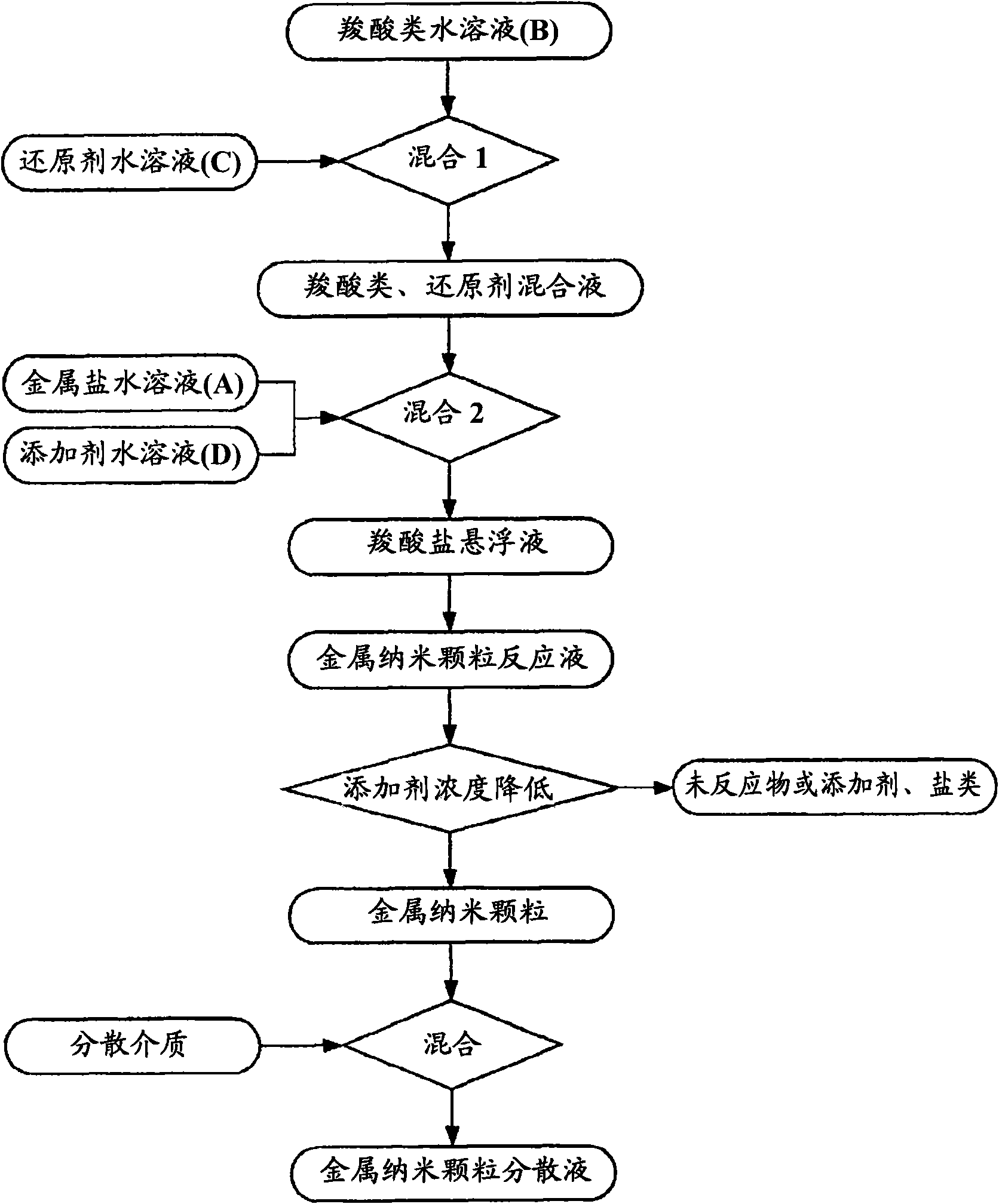
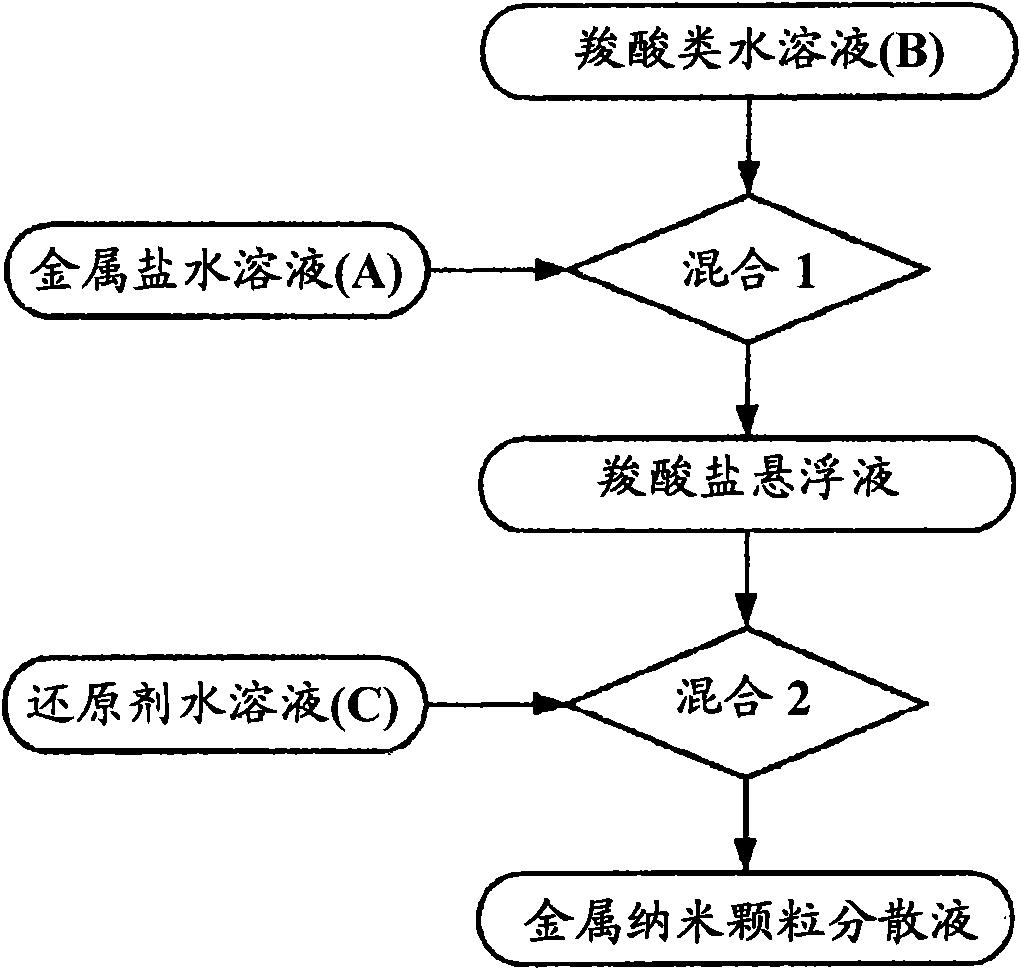
[0399] Next, the obtained carboxylic acid aqueous solution B and one of the metal salt aqueous solution A or the reducing agent aqueous solution C are mixed to form a mixed solution, and then any of the metal salt solution A or the reducing agent aqueous solution C is added to the mixed solution. A solution that has not been added, mixed further. There are two cases for this mixing order: if figure 1 As shown, first mix carboxylic acid aqueous solution B and metal salt aqueous solution A, then add reducing agent aqueous solution C in the mixed solution; also as figure 2 As shown, the carboxylic acid aqueous solution B and the reducing a...
Embodiment 21
[0425] First, silver nitrate was dissolved in deionized water to prepare a metal salt solution. Then chromium nitrate was dissolved in deionized water to prepare an additive aqueous solution. On the other hand, trisodium citrate is dissolved in deionized water, and in a nitrogen flow at a temperature of 35° C., granular ferrous sulfate is directly added to the trisodium citrate aqueous solution having a concentration of 26% to dissolve it, A carboxylic acid-reducing agent mixed solution containing citrate ions and ferrous ions at a molar ratio of 3:2 was prepared. Next, in the state where the above-mentioned nitrogen flow is maintained at a temperature of 35° C., the stirrer of the magnetic stirrer is rotated at a speed of 100 rpm, and the carboxylic acid-reductant mixed solution is stirred into the carboxylic acid-reductant mixed solution. The above-mentioned metal salt aqueous solution and additive aqueous solution were added dropwise and mixed. Wherein, the concentration ...
Embodiment 22
[0427] The chromium nitrate in Example 21 was replaced with tin nitrate, and the dispersion liquid obtained in the same manner as in Example 21 was left at room temperature, and aggregates of precipitated metal nanoparticles were separated by decantation. Deionized water was added to this isolate to prepare a dispersion liquid, which was subjected to desalination treatment by ultrafiltration, and then further continued to replace and wash with ethanol so that the metal content was 50% by mass. This dispersion liquid was used as the first dispersion liquid.
[0428] On the other hand, the silver nitrate in Example 21 was replaced by palladium nitrate, the chromium nitrate in Example 21 was replaced by tin nitrate, the dispersion obtained in the same manner as in Example 21 was left at room temperature, and the precipitated metal was separated by decantation. aggregates of nanoparticles. Deionized water was added to this isolate to prepare a dispersion liquid, which was subject...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com