New type antibiotic containing an antibody analog, preparation method and application method thereof
A technology of antibody mimics and antibiotics, which can be used in the field of biomedicine to solve problems such as bacterial mutation and drug resistance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] [Example 1] Construction of plasmids expressing novel antibiotics and preparation of novel antibiotics
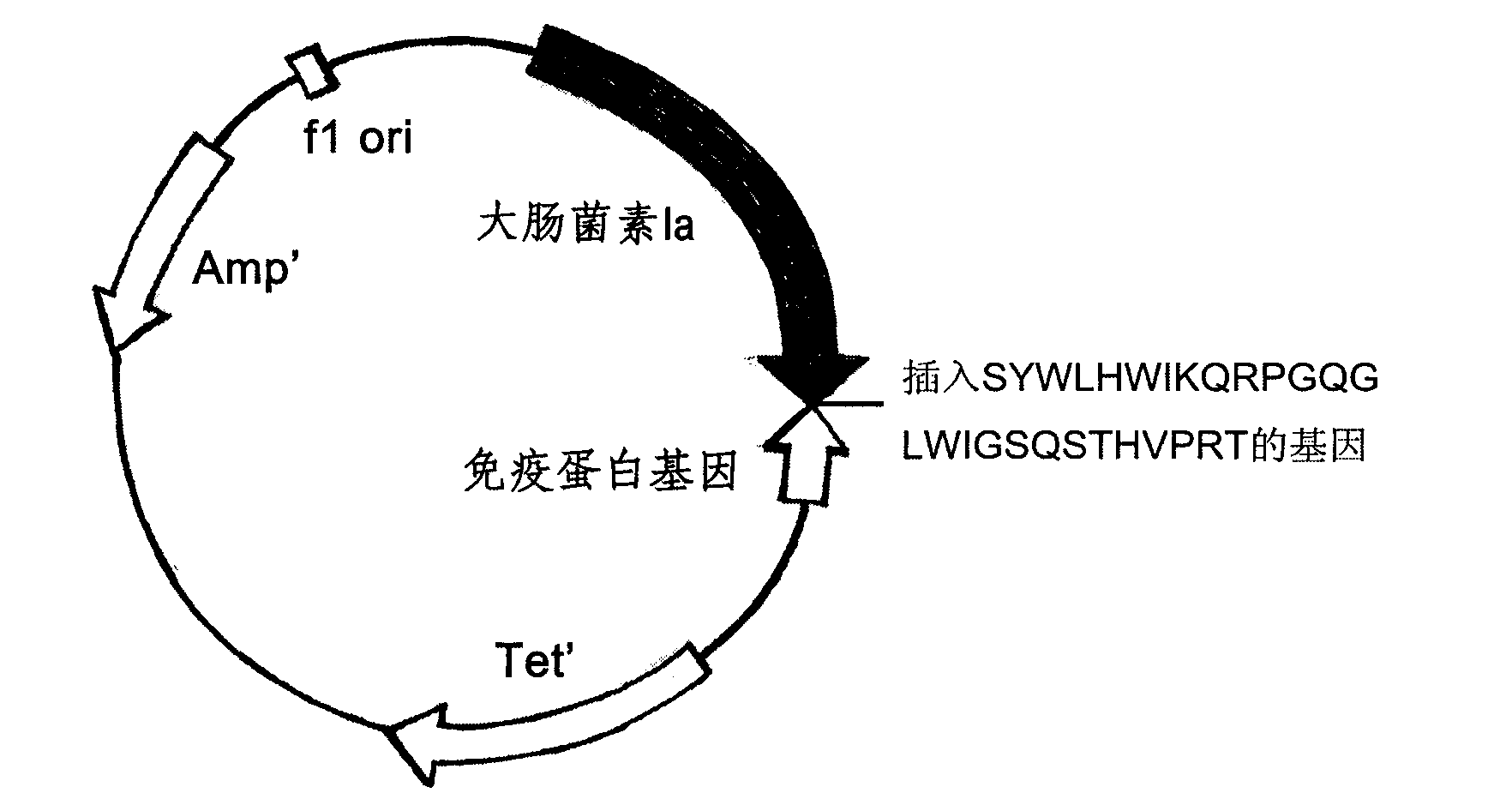
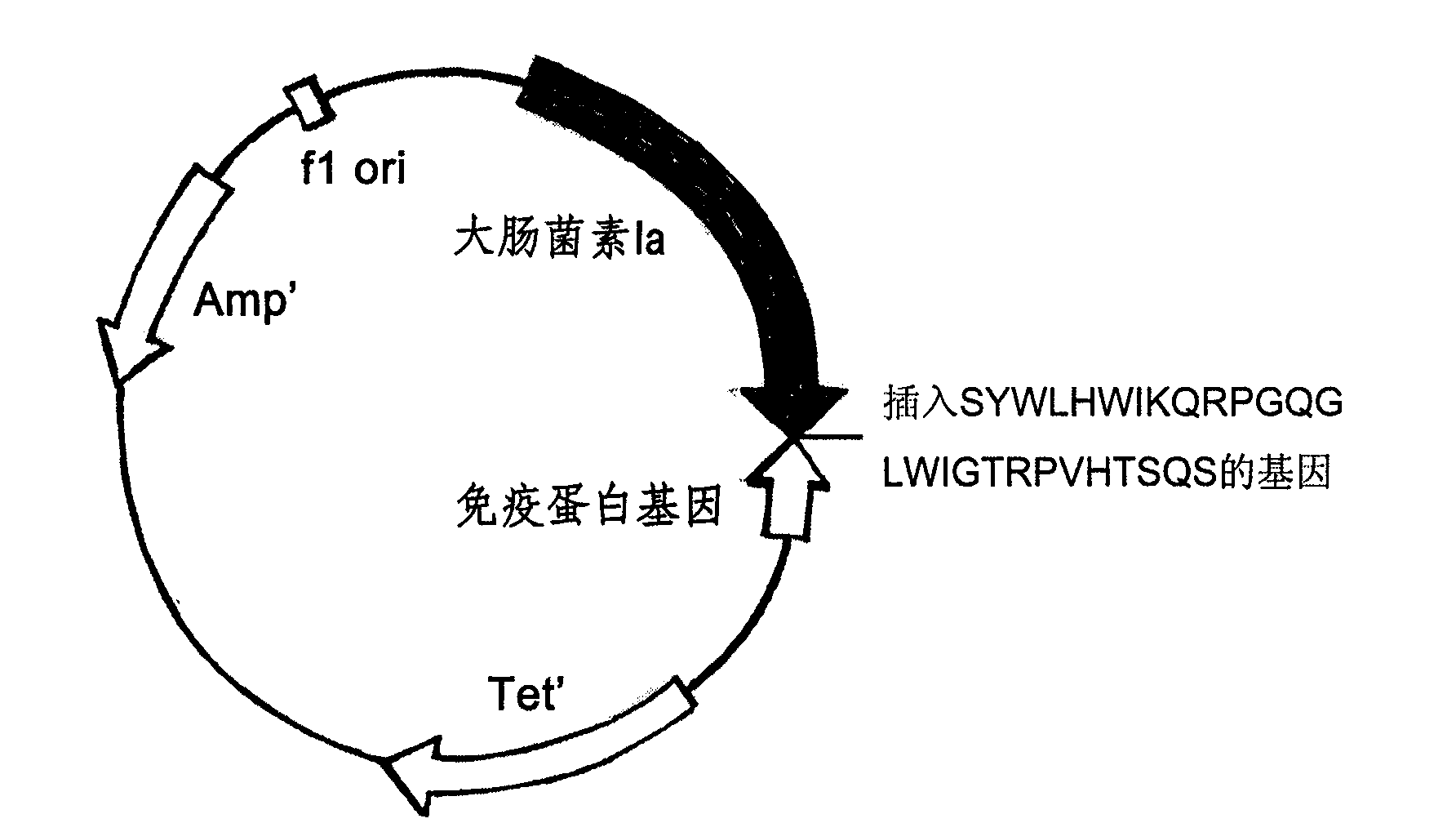
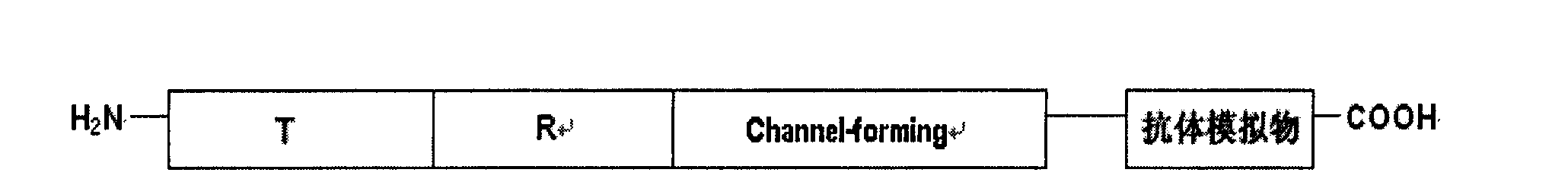
[0050] The original plasmid is pSELECT loaded with colicin and immunity protein genes TM -1 plasmid (8.3kb). Double-stranded oligonucleotide point mutation technology (QuickChange TM Kit, Strategene Company) will encode the antibody mimetic gene, such as the nucleotide sequence described in Seq ID No.1 and Seq ID No.3 respectively inserted on the 626 position of the colicin allosteric polypeptide gene, prepared a novel Antibiotic mutant plasmids pBHC-PorA1, pBHC-PorA2 (such as Figure 1-Figure 2 shown). The mutant plasmid was transfected into E.coli BL-21 engineering bacteria to prepare new antibiotics.
[0051] The mutation program was carried out according to the Strategene QuickChange SiteDirected Mutagenesis Kit (catalog#200518) kit manual: namely
[0052] 1. Prepare the point mutation reaction:
[0053] 5ul 10X buffer
[0054] 2ul (10ng) original plasmid...
Embodiment 2
[0103] [Example 2] The inhibitory effect of novel antibiotics on Neisseria meningitidis
[0104] The bacterium is the 29332 meningococcal strain of the China Culture Collection Center, and the bacterial solution is 2 microliters (10 5 CFU / ml) was added to 10 ml of rabbit blood chocolate culture solution (50 mg of beef extract, 100 mg of tryptone, KH 2 PO 4 30mg, NaCl 50mg, defibrillated rabbit blood 0.5-0.8ml), prepare 5 groups in total, add 0.3M NaCl+50mM boric acid buffer solution (that is, the blank preservation solution of new antibiotics, the amount is the same as the new antibiotics added in the experimental group) The amount of liquid is the same) as a control, the second group was added with 5 μg / ml ampicillin, the third group was added with 5 μg / ml PMC-AM1, the fourth group was added with 5 μg / ml PMC-AM2, and the fifth group was added with 10 μg / ml PMC-AM1.
[0105] The above-mentioned groups of liquids were respectively placed in 100 ml Erlenmeyer flasks, grown at ...
Embodiment 3
[0106] [Example 3] Comparison of the minimum inhibitory concentration of new antibiotics against multi-drug resistant Pseudomonas aeruginosa and the minimum inhibitory concentration of currently used antibiotics.
[0107] The minimum inhibitory concentration (MIC) of new antibiotics was determined by agar double dilution method. Bacteria were inoculated on the surface of agar plates containing different drug concentrations with a multi-point inoculation instrument (DeneleyA400), and the bacterial content of each point was 10 5 CFU / ml, incubate at 37°C for 18-24 hours to observe the results, and take the lowest concentration of the drug contained in the plate culture medium without bacteria as the minimum inhibitory concentration (MIC value) of the drug to the bacteria.
[0108] The bacterial species used is multi-drug resistant Pseudomonas aeruginosa (clinical isolate 13578 of West China Hospital), and the culture medium is MH medium (every 100 milliliters: 500 mg of beef extr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com