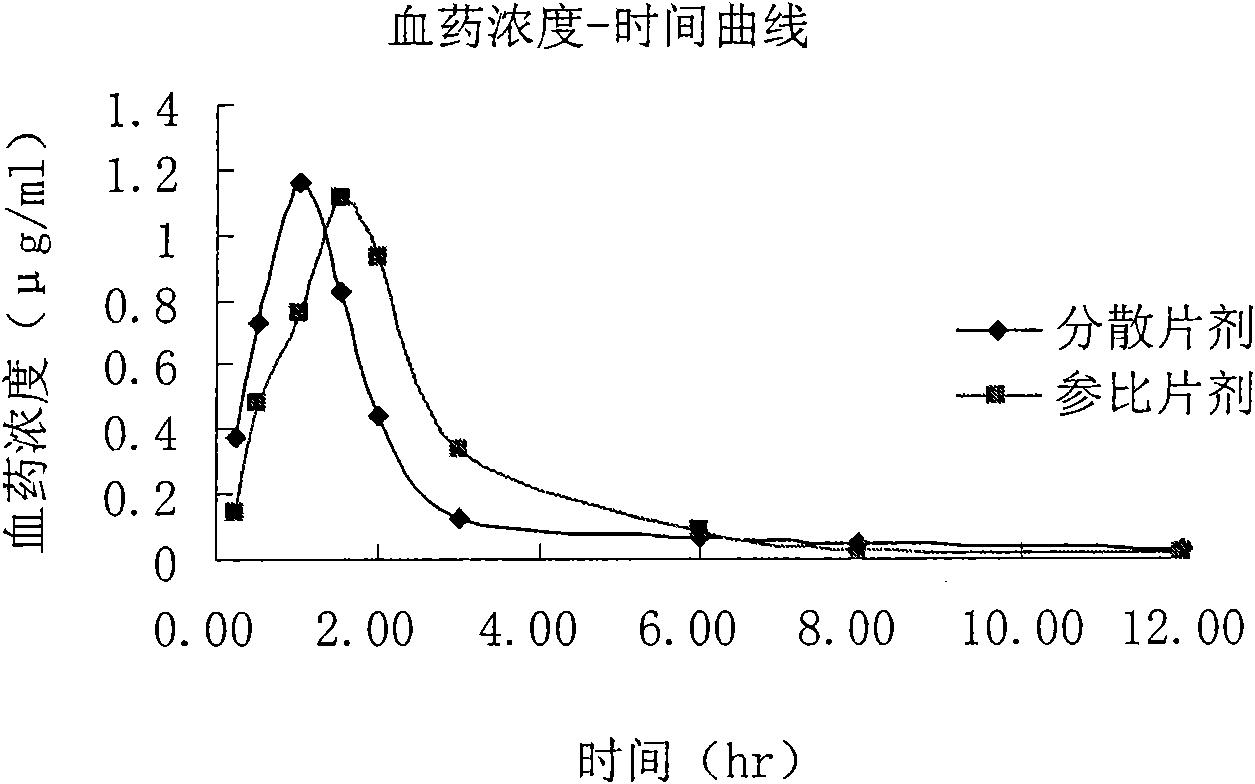
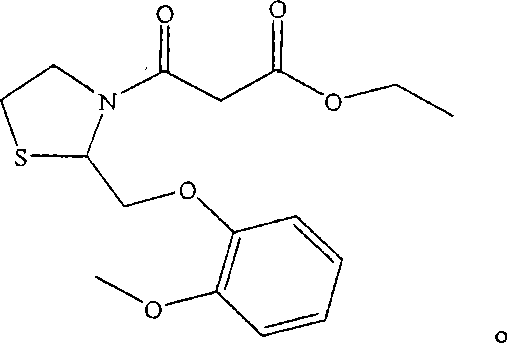
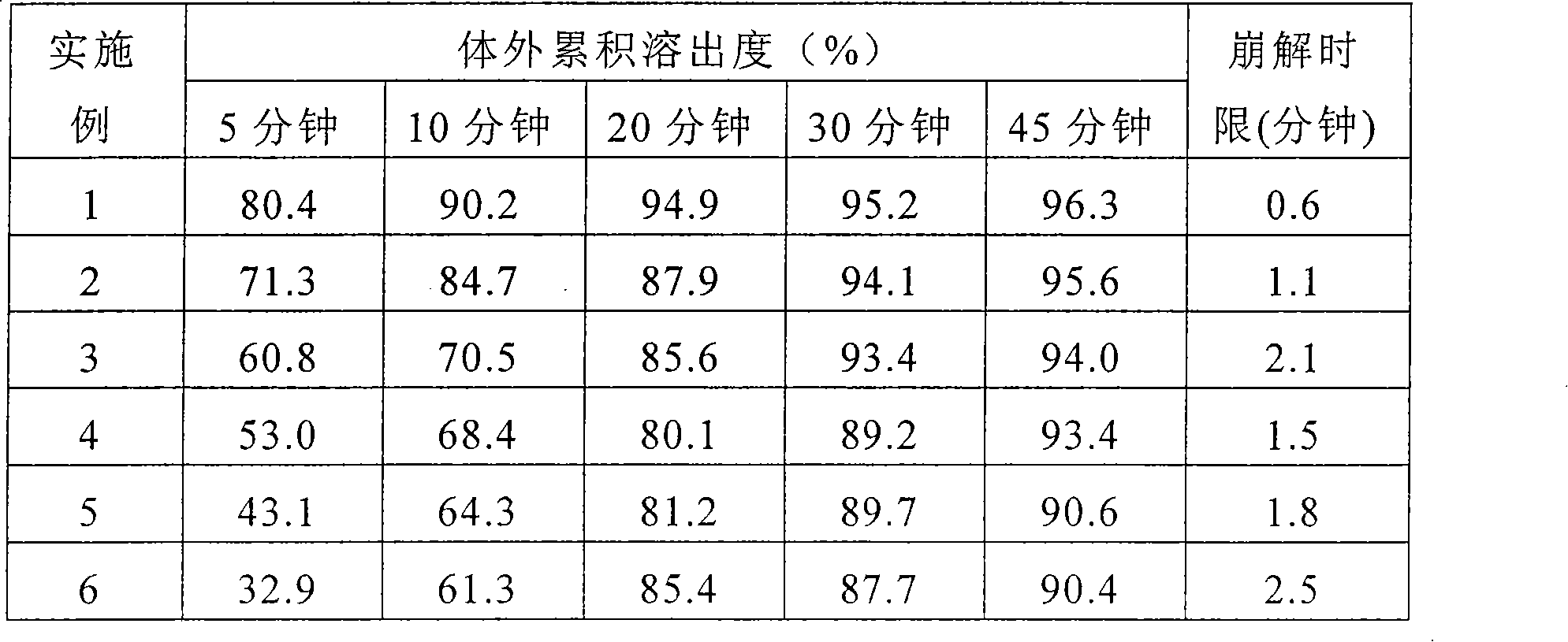
Moguisteine dispersible tablet and preparation method thereof
A technology for dispersible tablets within minutes, applied in the field of mogisteine dispersible tablets and its preparation, can solve the problems of low bioavailability and low solubility of oral solid preparations, achieve the goals of reducing adverse reactions, simple production process, and improving solubility Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] In another aspect, the present invention provides a method for preparing mogisteine dispersible tablets, which comprises the following steps: (1) mixing 5 parts by weight to 45 parts by weight of mogisteine, 10 parts by weight to 50 parts by weight The disintegrant, the filler of 20 parts by weight-80 parts by weight and the flavoring agent of 0.5 parts by weight-3.0 parts by weight are mixed to prepare a premix; (2) Add 0.5 parts by weight to 2.5 parts by weight of (3) granulating the obtained soft material; (4) drying the obtained granules; (5) sizing the dried granules; (6) adding 0.4 parts by weight to 1.5 parts by weight of Lubricants are blended and tabletted.
[0031] The preparation method of the present invention preferably further includes: before step (1), passing the mogisteine raw material through a 100-mesh sieve, and passing the disintegrating agent, filler, flavoring agent and lubricant through a 60-80 mesh sieve .
[0032] In the preparation metho...
Embodiment 1
[0050] First, pass the main ingredient of mogisteine through a 100-mesh sieve, and pass the excipients (including disintegrant sodium carboxymethyl starch, filler mannitol, flavoring agent aspartame and lubricant magnesium stearate) through a 60-mesh sieve. -80 mesh sieve.
[0051] Then, take by weighing 25g mogisteine, 50g sodium starch glycolate, 100g mannitol and 1.0g aspartame respectively, they are mixed by equal increment method, in the gained mixture, add 40ml of 3% (weight / volume) ethanol solution of povidone to make soft materials, pass through a 24-mesh sieve to granulate, and then air-dry at 40°C.
[0052] The dried granules are passed through a 24-mesh sieve for granulation, 1.6 g of magnesium stearate is added thereto, mixed evenly, and compressed into tablets to obtain the mogisteine dispersible tablet of the present invention.
[0053] Measure according to the method described in the two appendices of "Chinese Pharmacopoeia" (2005 edition), the disintegrat...
Embodiment 2
[0055] Prepare mogistein dispersible tablet according to the method described in embodiment 1, difference is to take by weighing 50g mogistein as main ingredient, take by weighing 20g cross-linked carmellose sodium and 50g cross-linked povidone As a disintegrant, weigh 80g mannitol and 100g pregelatinized starch as filler, weigh 1.2g aspartame as flavoring agent, and use 60ml of 5% (weight / volume) povidone ethanol solution as Binder, 3 g magnesium stearate as lubricant.
[0056] According to the method measurement described in the two appendices of "Chinese Pharmacopoeia" (2005 edition), the disintegration time limit of the dispersible tablet in this embodiment is 1.1 minutes.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com