Barium chloride production technology
A production process, barium chloride technology, applied in the direction of calcium/strontium/barium chloride, calcium/strontium/barium halide, etc., can solve the problem that impurities are difficult to completely remove, do not meet the needs of large-scale industries, and impurities are easy to enter, etc. problems, to achieve the effect of reducing pollution and waste of resources, adapting to large-scale industrial production, and low impurity content in products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
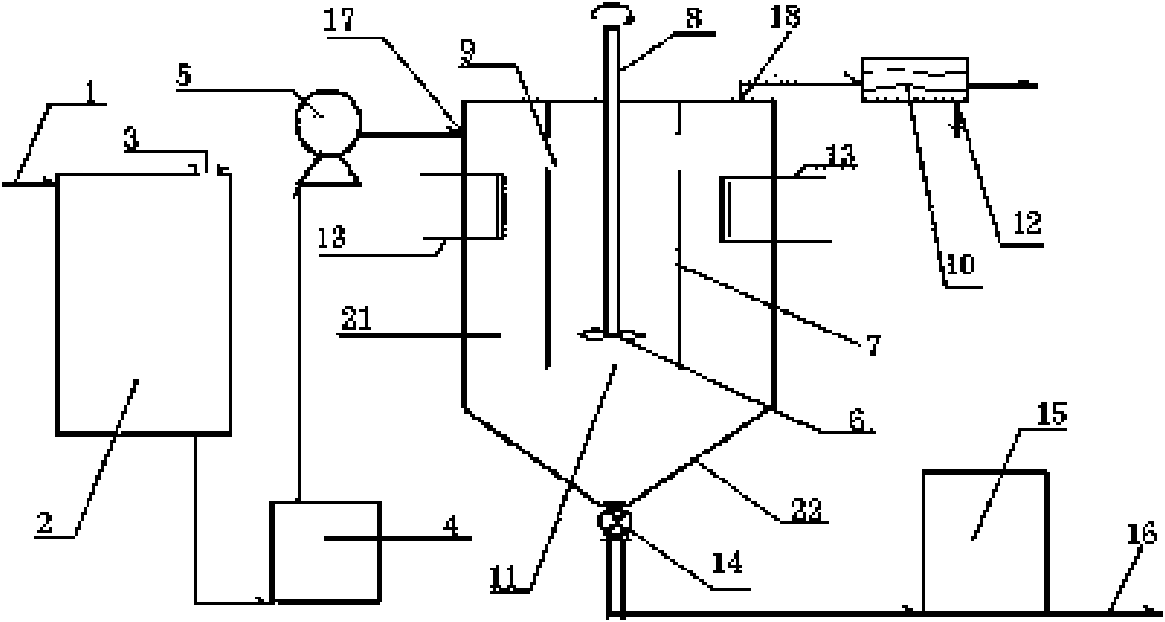
[0029] Embodiment 1: by witherite containing barium carbonate 65% by weight, by witherite: hydrochloric acid: water: hydrogen peroxide by 1: 0.37: 1: 0.1 ratio mixture 1 is input in reactor 2 at a constant speed, chemical reaction takes place, wherein hydrochloric acid The weight percent concentration is 30-32%, and the weight percent concentration of hydrogen peroxide is ≥ 29%. Free sulfur (S) and iron (Fe) undergo oxidation reaction with hydrogen peroxide and generate precipitates. The filter residue, the filtrate is barium chloride hydrate raw material solution, the released carbon dioxide is recovered and purified through outlet 3 to produce barium carbonate, and the filter residue is used as cement additive; Keep the temperature in the crystallization tank 21 at 100°C to 113°C, preferably 103°C. The vacuum condensation recovery device 10 sucks water vapor and condenses it for reuse. The vacuum is maintained at 10 to 30Kpa. Under the drive, in the crystallization kettle 21...
Embodiment 3
[0039] Embodiment 3: Repeat Example 1 according to the same steps as described, but witherite contains barium carbonate in 50% by weight, witherite: hydrochloric acid: water: hydrogen peroxide is mixed in a ratio of 1.3: 0.37: 1: 0.1 for a uniform input reaction In kettle 2, a chemical reaction takes place. Gained product barium chloride is identical with embodiment 1.
Embodiment 4
[0040] Embodiment 4: Repeat Example 1 according to the same steps as described, but witherite contains barium carbonate in 70% by weight, witherite: hydrochloric acid: water: hydrogen peroxide is mixed in a ratio of 0.93: 0.37: 1: 0.1 for 1 uniform input reaction In kettle 2, a chemical reaction takes place. Gained product barium chloride is identical with embodiment 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com