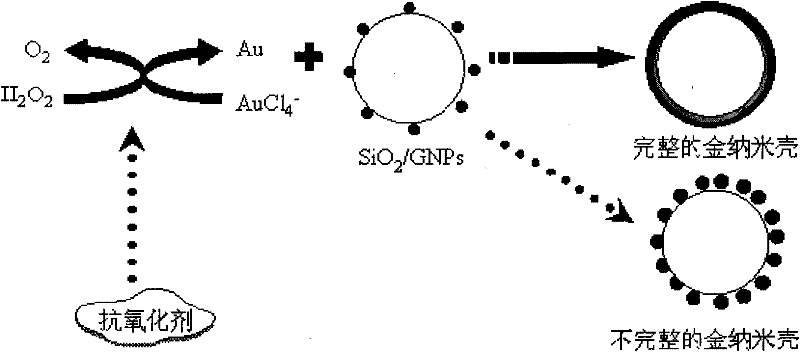
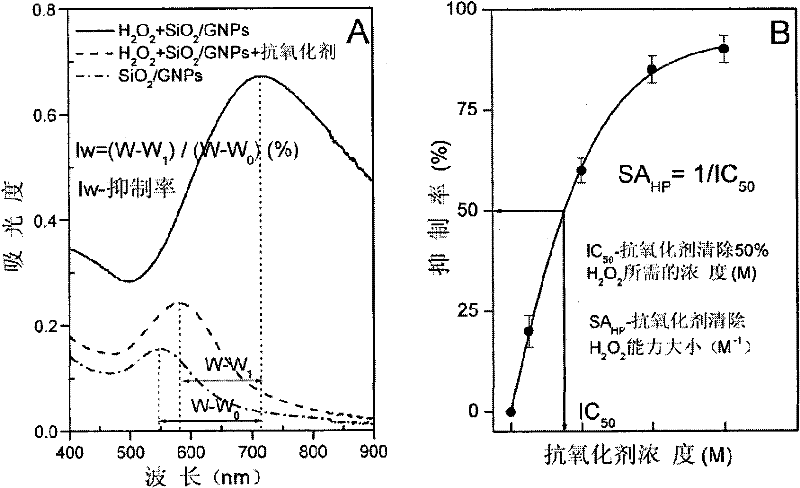
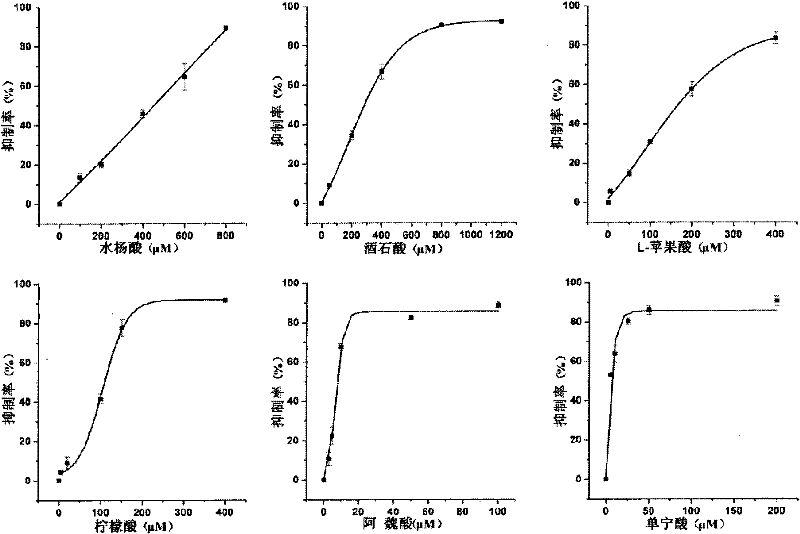
Gold nanoshell-based method for determining capability of antioxidant for clearing H2O2
An antioxidant, H2O2 technology, applied in the direction of chemical reaction of materials, material analysis by observing the impact on chemical indicators, etc., can solve problems such as carcinogenesis, achieve short operation time, wide detection range, preparation handy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] A gold nanoshell-based antioxidant for scavenging H 2 o 2 Ability to measure the method, the steps are:
[0032] 1. Incorporation of gold nanoparticles with aminated SiO 2 The colloidal solution is mixed and stirred, and the amount of the added gold nanoparticles should make the gold nanoparticles in the aminated SiO 2 The theoretically calculated value of coverage on the surface of the bead is not less than 30%. For example, 30%, 50%, 70%, and 90% are respectively selected in this embodiment, and the gold nanoparticles are adsorbed on the aminated SiO by electrostatic adsorption. 2 On the surface of colloidal particles, SiO 2 / GNPs composite particle aqueous solution, in the SiO 2 After centrifugation of the aqueous solution of / GNPs composite particles, the unadsorbed gold nanoparticles in the upper layer were removed, and then the SiO 2 / GNPs composite particle aqueous solution was diluted with pure water, and the precipitate was dispersed in pure water to obtai...
Embodiment 2
[0038] 1. Preparation of Gold Nanoparticles (GNPs)
[0039]Gold nanoparticles were prepared by reducing chloroauric acid. Dissolve 1 g of chloroauric acid in 100 mL of pure water to obtain a 1% chloroauric acid aqueous solution, and refrigerate at 4°C. Take 200mL of pre-cooled pure water, add 3mL of 1% chloroauric acid aqueous solution and 1mL of 0.2M potassium carbonate aqueous solution, stir and mix well, then quickly add 9mL of 0.5mg / mL sodium borohydride to it Aqueous solution, the mixed solution changes from dark brown to wine red to obtain gold nanoparticles with a particle size of about 5nm. After stirring for another 10 min, the prepared gold nanoparticle aqueous solution was stored at 4° C. in a refrigerator.
[0040] 2. SiO 2 colloidal amination
[0041] Take 40mL of SiO with a concentration of 0.013g / mL 2 Alcohol sol, add 60μL of γ-aminopropyltriethoxysilane (APTES) to it, stir and react at 70°C for 3h, after the reaction, put the above solution in a centrifuge...
Embodiment 3
[0051] 1. Same as embodiment 2 step 1.
[0052] 2. With embodiment 2 step 2.
[0053] 3. Same as step 3 of embodiment 2.
[0054] 4. Same as step 4 of embodiment 2.
[0055] 5. Tartaric acid scavenge H 2 o 2 Determination of ability
[0056] Select the final concentration of tartaric acid in the reaction system to be 1200 μ M, and all the other experimental operation steps are the same as step 5 of embodiment 2, and the tartaric acid of 1200 μ M is calculated to H 2 o 2 The clearance percentage is 92.5%. Reduce the concentration of tartaric acid so that the final concentrations in the reaction system are 800 μM, 400 μM, 200 μM, and 50 μM, respectively. Repeat the above steps to calculate the 800μM, 400μM, 200μM, 50μM tartaric acid to H 2 o 2 The clearance percentages were 90.1%, 62.9%, 36.7%, 9.1%.
[0057] 6. According to the above experiments, different concentrations of tartaric acid have different effects on H 2 o 2 The clearance rate data, using ORIGIN drawing...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| cover factor | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com